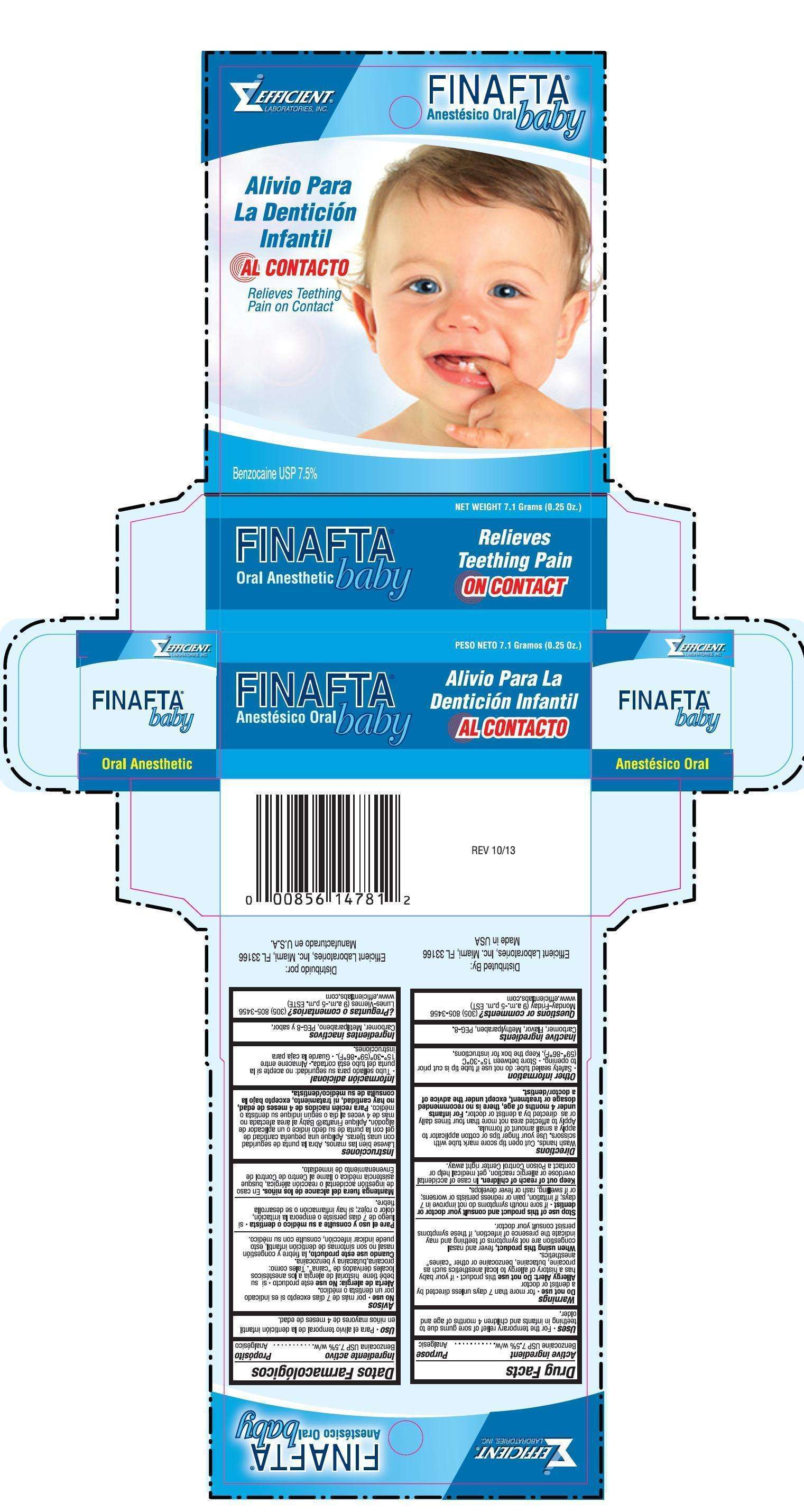
Finafta
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients: (%) Purpose
Benzocaine USP 7.5% w/w................ Analgesic
Purpose
Purpose
Analgesic
Uses
USES
For the temporary relief of sore gums due to teething in infants and children 4 months of age and older.
Warnings
When using this product fever and nasal congestion are not symptoms of teething and may indicate the presence of infection, if these symptoms persist, consult your doctor.
Do not use for more than 7 days unless directed by a dentist or doctor
Allergy Alert: Do not use this product if your baby has a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caines" anesthetics.
Stop use of this product and consult your doctor or dentist if sore mouth symptoms do not get better in 7 days; if irritation, pain or redness persists or worsens; or if swelling, rash or fever develops.
Keep out of reach of children. In case of accidental overdose or allergic reaction, get medical help or contact a Poison Control Center right away.
Directions
Wash hands. Cut open tip on score mark with scissors Use your fingertips or cotton applicator to apply a small pea size amount of formula. Apply to affected area not more than four times daily or as directed by a dentist or doctor For infants under 4 months of age, there is no recommended dosage or treatment, except under the advice of a doctor/dentist
Inactive ingredients Carbomer, flavor, Methylparaben and PEG-8
Questions or Comments?
1-305-805-3456
Monday-Friday (9 a.m. - 5 p.m. EST)
www.efficientlabs.com
Distributed by
Efficient Laboratories, Inc. Miami Fl 33166 USA
FinaftaBenzocaine LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||