Fexofenadine Hydrochloride
Bryant Ranch Prepack
Bryant Ranch Prepack
HIGHLIGHTS OF PRESCRIBING INFORMATION INDICATIONS AND USAGEFexofenadine hydrochloride USP is an H -receptor antagonist indicated for: 1 Relief of symptoms associated with seasonal allergic rhinitis in patients 6 years of age and older ( ) 1.1 Treatment of uncomplicated skin manifestations of chronic idiopathic urticaria in patients 6 years of age and older ( ) 1.2 DOSAGE AND ADMINISTRATION Patient Population fexofenadine hydrochloride tablets ( ) 2.1 Adults and children ≥ 12 years 60 mg twice daily , or 180 mg once daily starting dose in patients with decreased renal function should be the recommended dose indicated above but administered once daily dose not for use in patients with decreased renal function Children 6 to 11 years 30 mg twice daily Children 2 to 5 years N/A Children 6 months to less than 2 years N/A Fexofenadine hydrochloride tablets: take with water ( ) 2.1 DOSAGE FORMS AND STRENGTHS Fexofenadine hydrochloride tablets USP: 30 mg, 60 mg, and 180 mg ( ) 3 CONTRAINDICATIONSPatients with known hypersensitivity to fexofenadine and any of the ingredients of fexofenadine hydrochloride tablets. ( ) 4 WARNINGS AND PRECAUTIONSFexofenadine hydrochloride tablets do not contain phenylalanine.Side EffectsThe most common adverse reactions (≥ 2%) in subjects age 12 years and older were headache, back pain, dizziness, stomach discomfort, and pain in extremity. In subjects aged 6 to 11 years, cough, upper respiratory tract infection, pyrexia and otitis media were more frequently reported. In subjects aged 6 months to 5 years, vomiting, diarrhea, somnolence/fatigue and rhinorrhea were more frequently reported. (6.1). Other adverse reactions have been reported. ( ) 6 To report SUSPECTED ADVERSE REACTIONS, contact Dr. Reddy’s Laboratories, Inc. at 1-888-375-3784 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONS Antacids: Do not take at the same time as aluminum and magnesium containing antacids ( ) 7.1 Fruit juice: Take with water; not fruit juice USE IN SPECIFIC POPULATIONS Pregnancy: Use only if benefit justifies risk to fetus ( ) 8.1 Nursing Mothers: Use with caution ( ) 8.3
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 FEXOFENADINE HYDROCHLORIDE INDICATIONS AND USAGE
- 2 FEXOFENADINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 FEXOFENADINE HYDROCHLORIDE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 FEXOFENADINE HYDROCHLORIDE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 FEXOFENADINE HYDROCHLORIDE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 17. PATIENT COUNSELING INFORMATION
- Fexofenadine Hcl 60mg Tablet
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
Fexofenadine hydrochloride tablets USP are available as:
30 mg are pink colored, oval shaped tablets embossed with "192" on one side and "R" on the other side.
60 mg are pink colored, oval shaped tablets embossed with "193" on one side and "R" on the other side.
180 mg are pink colored, oval, beveled edged, biconvex tablets debossed "194" on one side and "R" on the other side.
4 CONTRAINDICATIONS
Fexofenadine hydrochloride tablets are contraindicated in patients with known hypersensitivity to fexofenadine and any of the ingredients of fexofenadine hydrochloride. Rare cases of hypersensitivity reactions with manifestations such as angioedema, chest tightness, dyspnea, flushing and systemic anaphylaxis have been reported.
5 WARNINGS AND PRECAUTIONS
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
It is not known if fexofenadine is excreted in human milk. There are no adequate and well-controlled studies in women during lactation. Because many drugs are excreted in human milk, caution should be exercised when fexofenadine hydrochloride is administered to a nursing woman.
8.4 Pediatric Use
The recommended doses of fexofenadine hydrochloride in pediatric patients 6 months to 11 years of age are based on cross-study comparison of the pharmacokinetics of fexofenadine in adults and pediatric subjects and on the safety profile of fexofenadine hydrochloride in both adult and pediatric subjects at doses equal to or higher than the recommended doses. The safety and effectiveness of fexofenadine hydrochloride in pediatric patients under 6 months of age have not been established.
The safety of fexofenadine hydrochloride is based on the administration of fexofenadine hydrochloride tablets at a dose of 30 mg twice daily demonstrated in 438 pediatric subjects 6 years to 11 years of age in 2 placebo-controlled 2-week seasonal allergic rhinitis trials. The safety of fexofenadine hydrochloride at doses of 15 mg and 30 mg given once and twice a day has been demonstrated in 969 pediatric subjects (6 months to 5 years of age) with allergic rhinitis in 3 pharmacokinetic studies and 3 safety studies. The safety of fexofenadine hydrochloride for the treatment of chronic idiopathic urticaria in subjects 6 months to 11 years of age is based on cross-study comparison of the pharmacokinetics of fexofenadine hydrochloride in adult and pediatric subjects and on the safety profile of fexofenadine in both adult and pediatric subjects at doses equal to or higher than the recommended dose.
The effectiveness of fexofenadine hydrochloride for the treatment of seasonal allergic rhinitis in subjects 6 to 11 years of age was demonstrated in 1 trial (n=411) in which fexofenadine hydrochloride tablets 30 mg twice daily significantly reduced total symptom scores compared to placebo, along with extrapolation of demonstrated efficacy in subjects aged 12 years and above, and the pharmacokinetic comparisons in adults and children. The effectiveness of fexofenadine hydrochloride 30 mg twice daily for the treatment of seasonal allergic rhinitis in patients 2 to 5 years of age is based on the pharmacokinetic comparisons in adult and pediatric subjects and an extrapolation of the demonstrated efficacy of fexofenadine hydrochloride in adult subjects with this condition and the likelihood that the disease course, pathophysiology, and the drug's effect are substantially similar in pediatric patients to those in adult patients. The effectiveness of fexofenadine hydrochloride for the treatment of chronic idiopathic urticaria in patients 6 months to 11 years of age is based on the pharmacokinetic comparisons in adults and children and an extrapolation of the demonstrated efficacy of fexofenadine hydrochloride in adults with this condition and the likelihood that the disease course, pathophysiology and the drug's effect are substantially similar in children to that of adult patients. Administration of a 15 mg dose of fexofenadine hydrochloride to pediatric subjects 6 months to less than 2 years of age and a 30 mg dose to pediatric subjects 2 to 11 years of age produced exposures comparable to those seen with a dose of 60 mg administered to adults.
8.5 Geriatric Use
Clinical studies of fexofenadine hydrochloride tablets and capsules did not include sufficient numbers of subjects aged 65 years and over to determine whether this population responds differently from younger subjects. Other reported clinical experience has not identified differences in responses between the geriatric and younger subjects. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see ]. Clinical Pharmacology (12.3)
10 OVERDOSAGE
Dizziness, drowsiness, and dry mouth have been reported with fexofenadine hydrochloride overdose. Single doses of fexofenadine hydrochloride up to 800 mg (6 healthy subjects at this dose level), and doses up to 690 mg twice daily for 1 month (3 healthy subjects at this dose level) or 240 mg once daily for 1 year (234 healthy subjects at this dose level) were administered without the development of clinically significant adverse events as compared to placebo.
In the event of overdose, consider standard measures to remove any unabsorbed drug. Symptomatic and supportive treatment is recommended. Following administration of terfenadine, hemodialysis did not effectively remove fexofenadine, the major active metabolite of terfenadine, from blood (up to 1.7% removed).
No deaths occurred at oral doses of fexofenadine hydrochloride up to 5000 mg/kg in mice (110 times the maximum recommended daily oral dose in adults and children based on mg/m ) and up to 5000 mg/kg in rats (230 times the maximum recommended daily oral dose in adults and 210 times the maximum recommended daily oral dose in children based on mg/m ). Additionally, no clinical signs of toxicity or gross pathological findings were observed. In dogs, no evidence of toxicity was observed at oral doses up to 2000 mg/kg (300 times the maximum recommended daily oral dose in adults and 280 times the maximum recommended daily oral dose in children based on mg/m ). 2 2 2
11 DESCRIPTION
Fexofenadine hydrochloride, the active ingredient of fexofenadine hydrochloride tablets, is a histamine H -receptor antagonist with the chemical name (±)-4-[1 hydroxy-4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-butyl]-α, α-dimethyl benzeneacetic acid hydrochloride. It has the following chemical structure 1
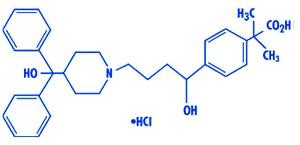
The molecular weight is 538.13 and the molecular formula is C H NO •HCl. 32 39 4
Fexofenadine hydrochloride USP is a white to off-white crystalline powder. It is freely soluble in methanol and ethanol, slightly soluble in chloroform and water, and insoluble in hexane. Fexofenadine hydrochloride is a racemate and exists as a zwitterion in aqueous media at physiological pH.
Fexofenadine hydrochloride USP is formulated as a tablet for oral administration. Each tablet contains 30, 60, or 180 mg fexofenadine hydrochloride (depending on the dosage strength) and the following excipients: colloidal silicon dioxide, corn starch, croscarmellose sodium, magnesium stearate, mannitol, and powder cellulose. The aqueous tablet film coating is made from (Opadry Pink 03B54504) containing FD&C Red no. 40, hypromellose 2910 6cP, iron oxide black, polyethylene glycol 400 and titanium dioxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fexofenadine hydrochloride, the major active metabolite of terfenadine, is an antihistamine with selective H -receptor antagonist activity. Both enantiomers of fexofenadine hydrochloride displayed approximately equipotent antihistaminic effects. Fexofenadine hydrochloride inhibited antigen-induced bronchospasm in sensitized guinea pigs and histamine release from peritoneal mast cells in rats. The clinical significance of these findings is unknown. In laboratory animals, no anticholinergic or alpha -adrenergic blocking effects were observed. Moreover, no sedative or other central nervous system effects were observed. Radiolabeled tissue distribution studies in rats indicated that fexofenadine does not cross the blood-brain barrier. 1 1
12.2 Pharmacodynamics
12.3 Pharmacokinetics
The pharmacokinetics of fexofenadine hydrochloride in subjects with seasonal allergic rhinitis and subjects with chronic urticaria were similar to those in healthy subjects.
13 NONCLINICAL TOXICOLOGY
13. 1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of fexofenadine was assessed using terfenadine studies with adequate fexofenadine exposure (based on plasma area-under-the-concentration vs. time [AUC] values). No evidence of carcinogenicity was observed in an 18-month study in mice and in a 24-month study in rats at oral doses up to 150 mg/kg of terfenadine (which led to fexofenadine exposures that were approximately 3 and 5 times the exposure at the maximum recommended daily oral dose of fexofenadine hydrochloride in adults [180 mg] and children [60 mg] respectively).
In (Bacterial Reverse Mutation, CHO/HGPRT Forward Mutation, and Rat Lymphocyte Chromosomal Aberration assays) and (Mouse Bone Marrow Micronucleus assay) tests, fexofenadine hydrochloride revealed no evidence of mutagenicity. in vitro in vivo
In rat fertility studies, dose-related reductions in implants and increases in postimplantation losses were observed at an oral dose of 150 mg/kg of terfenadine (which led to fexofenadine exposures that were approximately 3 times the exposure at the maximum recommended human daily oral dose of 180 mg of fexofenadine hydrochloride based on comparison of AUCs). In mice, fexofenadine hydrochloride produced no effect on male or female fertility at average oral doses up to 4438 mg/kg (which led to fexofenadine exposures that were approximately 13 times the exposure at the maximum recommended human daily oral dose of 180 mg of fexofenadine hydrochloride based on comparison of AUCs).
14 CLINICAL STUDIES
17. PATIENT COUNSELING INFORMATION
Provide the following information to patients and parents/caregivers of pediatric patients taking fexofenadine hydrochloride tablets:
- Fexofenadine hydrochloride tablets, are prescribed for the relief of symptoms of seasonal allergic rhinitis or for the relief of symptoms of chronic idiopathic urticaria (hives). Instruct patients to take fexofenadine hydrochloride only as prescribed. . If any untoward effects occur while taking fexofenadine hydrochloride, discontinue use and consult a doctor. Do not exceed the recommended dose
- Patients who are hypersensitive to any of the ingredients should not use these products.
- Patients who are pregnant or nursing should use these products only if the potential benefit justifies the potential risk to the fetus or nursing infant.
- Advise patients and parents/caregivers of pediatric patients to store the medication in a tightly closed container in a cool, dry place, away from small children.
- Advise patients and parents/caregivers not to take fexofenadine hydrochloride tablets with fruit juices.
For fexofenadine hydrochloride tablets: Advise patients to take the fexofenadine hydrochloride tablets with water.
Rx only
Manufactured by: Bachepalli – 502 325 INDIA
Dr. Reddy's Laboratories Limited
Revised: 0610
Fexofenadine Hcl 60mg Tablet

Fexofenadine HydrochlorideFexofenadine Hydrochloride TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||