FENTANYL
HIGHLIGHTS OF PRESCRIBING INFORMATION BOXED WARNING WARNING: ABUSE POTENTIAL, RESPIRATORY DEPRESSION AND DEATH, ACCIDENTAL EXPOSURE, CYTOCHROME P450 3A4 INTERACTION, AND EXPOSURE TO HEAT See full prescribing information for complete boxed warning Contains a high concentration of fentanyl, a Schedule II controlled substance, which is subject to misuse, abuse, addiction, and criminal diversion. (9) Fatal respiratory depression could occur in patients who are not opioid‑tolerant and in patients that are opioid‑tolerant even if fentanyl transdermal system is not misused or abused. (5) Accidental exposure of fentanyl transdermal system, especially in children, can result in a fatal overdose of fentanyl. (5) CYP 3A4 inhibitors can result in a fatal overdose of fentanyl from fentanyl transdermal system. (5) Avoid exposing the fentanyl transdermal system application site and surrounding area to direct external heat sources. Temperature dependent increases in fentanyl releasefrom the system may result in overdose and death. (5) RECENT MAJOR CHANGESBoxed Warning 07/2012Indications and Usage (1) 07/2012Dosage and Administration (2) 07/2012Contraindications (4) 07/2012Warnings and Precautions (5) 07/2012INDICATIONS AND USAGE Fentanyl transdermal system contains fentanyl, a full opioid agonist. Fentanyl transdermal system is indicated for the management of persistent, moderate to severe chronic pain in opioid‑tolerant patients 2 years of age and older when a continuous, around-the-clock opioid analgesic is needed for an extended period of time. (1) Fentanyl transdermal system is NOT intended for use as an as-needed analgesic. (1) DOSAGE AND ADMINISTRATION Individualize treatment in every case as part of a pain management plan. (2) Initial dose selection: carefully select initial dose based on the status of each patient, consult conversion instructions. (2.1) Each transdermal system is intended to be worn for 72 hours. (2.2) Individually titrate to a tolerable dose that provides adequate analgesia. (2.2) Adhere to instructions concerning administration and disposal of fentanyl transdermal system. (2.3) When fentanyl transdermal system is no longer needed by the patient, taper the dose as part of a pain management plan. (2.4) Use with caution in hepatic and renally impaired patients. (2.2) DOSAGE FORMS AND STRENGTHS Transdermal system: 25 mcg/h, 50 mcg/h, 75 mcg/h, 100 mcg/h. (3) CONTRAINDICATIONS Opioid non‑tolerant patients. (4) Impaired pulmonary function. (4) Paralytic ileus. (4) Known hypersensitivity to fentanyl or any of the components of the transdermal system. (4) WARNINGS AND PRECAUTIONS Fentanyl transdermal system can be abused. Use caution when prescribing if there is an increased risk of misuse, abuse, or diversion. (5.1) Fatal respiratory depression can occur with fentanyl transdermal system. Monitor patients accordingly. Use with extreme caution in patients at risk of respiratory depression. (5.2) Accidental exposure of fentanyl transdermal system, especially in children, can result in a fatal overdose of fentanyl. (5.3) Use fentanyl transdermal system with extreme caution in patients susceptible to intracranial effects of CO2 retention. (5.6) Fentanyl transdermal systemmay have additive effects when used in conjunction with other CNS depressants, alcohol, and drugs of abuse. (5.7) Use of fentanyl transdermal system with a CYP3A4 inhibitor may result in an increase in fentanyl plasma concentrations. Monitor patients accordingly and adjust dosage if necessary. (5.8) Fentanyl transdermal system may produce bradycardia. Administer with caution to patients with bradyarrhythmias. (5.11) Use fentanyl transdermal system with caution in patients with pancreatic/biliary disease. (5.14) Side EffectsThe most common adverse reactions (≥5%) in a double-blind, randomized, placebo-controlled clinical trial in patients with severe pain were nausea, vomiting, somnolence, dizziness, insomnia, constipation, hyperhidrosis, fatigue, feeling cold, and anorexia. Other common adverse reactions (≥5%) reported in clinical trials in patients with chronic malignant or nonmalignant pain were headache and diarrhea. (6.0) To report SUSPECTED ADVERSE REACTIONS, call Apotex Corp. at 1-888-333-6127 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Monitor patients receiving fentanyl transdermal system and any CYP3A4 inhibitor for an extended period of time and adjust dosage, if necessary. (7.1) Use CNS Depressants with caution and in reduced dosage in patients who are receiving fentanyl transdermal system. (7.2) Avoid fentanyl transdermal system in patients taking a monoamine oxidase (MAO) inhibitor or within 14 days of stopping such treatment. (7.3) USE IN SPECIFIC POPULATIONS Pregnancy: Based on animal data, may cause fetal harm. (8.1) Nursing Mothers: Breast-feeding is not advised in mothers treated with fentanyl transdermal system. (8.3) Pediatric Use: Safety and efficacy in pediatric patients below the age of 2 years have not been established. To guard against accidental ingestion by children, use caution when choosing the application site for fentanyl transdermal system. (8.4) Geriatric Use: Administer fentanyl transdermal system with caution, and in reduced dosages in elderly patients. (8.5) Hepatic or Renal Impairment: Administer fentanyl transdermal system with caution. Monitor for signs of fentanyl toxicity and reduce dosage, if necessary. (8.6, 8.7) See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- RECENT MAJOR CHANGES SECTION
- 1 FENTANYL INDICATIONS AND USAGE
- 2 FENTANYL DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 FENTANYL CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 5.1 Abuse Potential
- 5.2 Respiratory Depression and Death
- 5.3 Accidental Exposure
- 5.4 Elderly, Cachectic, and Debilitated Patients
- 5.5 Chronic Pulmonary Disease
- 5.6 Head Injuries and Increased Intracranial Pressure
- 5.7 Interactions with Other CNS Depressants, Alcohol, and Drugs of Abuse
- 5.8 Interactions with CYP3A4 Inhibitors
- 5.9 Application of External Heat
- 5.10 Patients with Fever
- 5.11 Cardiac Disease
- 5.12 Hepatic Impairment
- 5.13 Renal Impairment
- 5.14 Use in Pancreatic/Biliary Tract Disease
- 5.15 Avoidance of Withdrawal
- 5.16 Driving and Operating Machinery
- 6 FENTANYL ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 FENTANYL DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
BOXED WARNING
WARNING: ABUSE POTENTIAL, RESPIRATORY DEPRESSION AND DEATH, ACCIDENTAL EXPOSURE, CYTOCHROME P450 3A4 INTERACTION, AND EXPOSURE TO HEAT
Abuse Potential
Fentanyl transdermal system contains fentanyl, an opioid agonist and a Schedule II controlled substance with an abuse liability similar to other opioid analgesics. Fentanyl transdermal system can be abused in a manner similar to other opioid agonists, legal or illicit. Persons at increased risk for opioid abuse include those with a personal or family history of substance abuse (including drug or alcohol abuse or addiction) or mental illness (e.g., major depression). Assess patients for their clinical risks for opioid abuse or addiction prior to prescribing fentanyl transdermal system and then routinely monitor all patients for signs of misuse, abuse and addiction during treatment [see Warnings and Precautions (5.1) and Drug Abuse and Dependence (9)].
Respiratory Depression and Death
Respiratory depression and death may occur with use of fentanyl transdermal system, even when fentanyl transdermal system has been used as recommended and not misused or abused. Proper dosing and titration are essential and fentanyl transdermal system should only be prescribed by healthcare professionals who are knowledgeable in the use of potent opioids for the management of chronic pain. Fentanyl transdermal system is contraindicated for use in conditions in which the risk of life-threatening respiratory depression is significantly increased, including use as an as-needed analgesic, use in non-opioid tolerant patients, acute pain, and postoperative pain. Monitor for respiratory depression, especially during the first two applications following initiation of dosing, or following an increase in dosage [see Contraindications (4) and Warnings and Precautions (5.2)].
Accidental Exposure
Death and other serious medical problems have occurred when children and adults were accidentally exposed to fentanyl transdermal system. Advise patients about strict adherence to the recommended handling and disposal instructions in order to prevent accidental exposure [see Dosage and Administration (2.3) (2.4) and Warnings and Precautions (5.3)].
Cytochrome P450 3A4 Interaction
The concomitant use of fentanyl transdermal system with all cytochrome P450 3A4 inhibitors may result in an increase in fentanyl plasma concentrations, which could increase or prolong adverse drug effects and may cause potentially fatal respiratory depression. Monitor patients receiving fentanyl transdermal system and any CYP3A4 inhibitor [see Warnings and Precautions (5.8), and Clinical Pharmacology (12.3)].
Exposure To Heat
The fentanyl transdermal system application site and surrounding area must not be exposed to direct external heat sources, such as heating pads or electric blankets, heat or tanning lamps, sunbathing, hot baths, saunas, hot tubs, and heated water beds. Exposure to heat may increase fentanyl absorption and there have been reports of overdose and death as a result of exposure to heat ( 5.9 ). Patients wearing fentanyl transdermal systems who develop fever or increased core body temperature due to strenuous exertion are also at risk for increased fentanyl exposure and may require an adjustment in the dose of fentanyl transdermal system to avoid overdose and death ( 5.10 ).
RECENT MAJOR CHANGES SECTION
1 INDICATIONS AND USAGE
Fentanyl transdermal system is a transdermal formulation of fentanyl indicated for the management of persistent, moderate to severe chronic pain in opioid-tolerant patients 2 years of age and older when a continuous, around-the-clock opioid analgesic is required for an extended period of time, and the patient cannot be managed by other means such as non-steroidal analgesics, opioid combination products, or immediate-release opioids.
Patients considered opioid-tolerant are those who are taking at least 60 mg of morphine daily, or at least 30 mg of oral oxycodone daily, or at least 8 mg of oral hydromorphone daily, or an equianalgesic dose of another opioid for a week or longer.
2 DOSAGE AND ADMINISTRATION
2.1Proper Patient Selection
Abuse Potential
Assess patients for their clinical risks for opioid abuse or addiction prior to being prescribed fentanyl transdermal system [see Warnings and Precautions (5.1)].
Opioid Tolerance
Opioid tolerance to an opioid of comparable potency must be established before prescribing fentanyl transdermal system [see Warnings and Precautions (5.2)].Patients considered opioid-tolerant are those who are taking at least 60 mg of morphine daily, or at least 30 mg of oral oxycodone daily, or at least 8 mg of oral hydromorphone daily or an equianalgesic dose of another opioid for a week or longer.
2.2 Dosing
Conversion to Fentanyl Transdermal System in Opioid-Tolerant Patients
The recommended starting dose when converting from other opioids to fentanyl transdermal system is intended to minimize the potential for overdosing patients with the first dose. Monitor patients closely for respiratory depression, especially within the first 24 to 72 hours of initiating therapy with fentanyl transdermal system [see Warnings and Precautions (5.2)].
In selecting an initial fentanyl transdermal system dose, take the following factors into account:
- the daily dose, potency, and characteristics of the opioid the patient has been taking previously (e.g., whether it is a pure agonist or mixed agonist-antagonist);
- the reliability of the relative potency estimates used to calculate the fentanyl transdermal system dose needed (potency estimates may vary with the route of administration);
- the degree of opioid tolerance;
- the general condition and medical status of the patient.
To convert adult and pediatric patients from oral or parenteral opioids to fentanyl transdermal system, use Table 1. Do not use Table 1 to convert from fentanyl transdermal system to other therapies because this conversion to fentanyl transdermal system is conservative and will overestimate the dose of the new agent.
|
TABLE 11
|
||||
| Current Analgesic | Daily Dosage (mg/day) | |||
| Oral morphine | 60-134 | 135-224 | 225-314 | 315-404 |
| Intramuscular or Intravenous morphine | 10-22 | 23-37 | 38-52 | 53-67 |
| Oral oxycodone | 30-67 | 67.5-112 | 112.5-157 | 157.5-202 |
| Oral codeine | 150-447 | |||
| Oral hydromorphone | 8-17 | 17.1-28 | 28.1-39 | 39.1-51 |
| Intravenous hydromorphone | 1.5-3.4 | 3.5-5.6 | 5.7-7.9 | 8-10 |
| Intramuscular meperidine | 75-165 | 166-278 | 279-390 | 391-503 |
| Oral methadone | 20-44 | 45-74 | 75-104 | 105-134 |
| ß | ß | ß | ß | |
| Recommended Fentanyl Transdermal System Dose | 25 mcg/hour | 50 mcg/hour | 75 mcg/hour | 100 mcg/hour |
| Alternatively, for adult and pediatric patients taking opioids or doses not listed in Table 1, use the conversion methodology outlined above with Table 2. 1Table 1 should not be used to convert from fentanyl transdermal system to other therapies because this conversion to fentanyl transdermal system is conservative.Use of Table 1 for conversion to other analgesic therapies can overestimate the dose of the new agent.Overdosage of the new analgesic agent is possible [ see Dosage and. Administration (2.3) ] |
||||
Alternatively, for adult and pediatric patients taking opioids or doses not listed in Table 1, use the following methodology:
- Calculate the previous 24-hour analgesic requirement.
- Convert this amount to the equianalgesic oral morphine dose using a reliable reference.
Refer to Table 2 for the range of 24-hour oral morphine doses that are recommended for conversion to each fentanyl transdermal system dose. Use this table to find the calculated 24‑hour morphine dose and the corresponding fentanyl transdermal system dose. Initiate fentanyl transdermal system treatment using the recommended dose and titrate patients upwards (no more frequently than 3 days after the initial dose and every 6 days thereafter) until analgesic efficacy is attained. - Do not use Table 2 to convert from fentanyl transdermal system to other therapies because this conversion to fentanyl transdermal system is conservative and will overestimate the dose of the new agent.
|
TABLE 2
1
|
|
| Oral 24-hourMorphine (mg/day) | Fentanyl Transdermal SystemDose (mcg/hour) |
| 60-134 | 25 |
| 135-224 | 50 |
| 225-314 | 75 |
| 315-404 | 100 |
| 405-494 | 125 |
| 495-584 | 15 |
| 585-674 | 175 |
| 675-764 | 200 |
| 765-854 | 225 |
| 855-944 | 250 |
| 945-1034 | 275 |
| 1035-1124 | 300 |
| NOTE: In clinical trials, these ranges of daily oral morphine doses were used as a basis for conversion to fentanyl transdermal system. 1Table 2 should not be used to convert from fentanyl transdermal system to other therapies because this conversion to fentanyl transdermal system is conservative. Use of Table 2 for conversion to other analgesic therapies can overestimate the dose of the new agent. Overdosage of the new analgesic agent is possible [see Dosage and Administration (2.3)]. |
|
For delivery rates in excess of 100 mcg/hour, multiple systems may be used.
Hepatic Impairment
Avoid the use of fentanyl transdermal system in patients with severe hepatic impairment. In patients with mild to moderate hepatic impairment, start with one half of the usual dosage of fentanyl transdermal system. Closely monitor for signs of sedation and respiratory depression, including at each dosage increase [see Warnings and Precautions (5.12), Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Renal Impairment
Avoid the use of fentanyl transdermal system in patients with severe renal impairment. In patients with mild to moderate renal impairment, start with one half of the usual dosage of fentanyl transdermal system. Closely monitor for signs of sedation and respiratory depression, including at each dosage increase [see Warnings and Precautions (5.13), Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
2.3 Titration and Maintenance of Therapy
Once therapy is initiated, assess pain intensity and opioid adverse reactions frequently, especially respiratory depression [see Warnings and Precautions (5.2)]. Routinely monitor all patients for signs of misuse, abuse and addiction [see Warnings and Precautions (5.1)].
The initial fentanyl transdermal system dose may be increased after 3 days based on the daily dose of supplemental opioid analgesics required by the patient on the second or third day of the initial application.
It may take up to 6 days for fentanyl levels to reach equilibrium on a new dose [see Clinical Pharmacology (12.3)]. Therefore, evaluate patients for further titration after no less than two 3-day applications before any further increase in dosage is made.
Base dosage increments on the daily dosage of supplementary opioids, using the ratio of 45 mg/24 hours of oral morphine to a 12 mcg/hour increase in fentanyl transdermal system dose.
The majority of patients are adequately maintained with fentanyl transdermal system administered every 72 hours. Some patients may not achieve adequate analgesia using this dosing interval and may require systems to be applied at 48 hours rather than at 72 hours, only if adequate pain control cannot be achieved using a 72-hour regimen. An increase in the fentanyl transdermal system dose should be evaluated before changing dosing intervals in order to maintain patients on a 72‑hour regimen. Dosing intervals less than every 72 hours were not studied in children and adolescents and are not recommended.
Discontinuation of Fentanyl Transdermal System
To convert patients to another opioid, remove fentanyl transdermal system and titrate the dose of the new analgesic based upon the patient’s report of pain until adequate analgesia has been attained. Upon system removal, 17 hours or more are required for a 50% decrease in serum fentanyl concentrations. Withdrawal symptoms are possible in some patients after conversion or dose adjustment [see Warnings and Precautions (5.15)].
Do not use Tables 1 and 2 to convert from fentanyl transdermal system to other therapies to avoid overestimating the dose of the new agent potentially resulting in overdose of the new analgesic and death.
When discontinuing fentanyl transdermal system and not converting to another opioid, use a gradual downward titration, such as halving the dose every 6 days, in order to reduce the possibility of withdrawal symptoms [see Warnings and Precautions (5.15)]. It is not known at what dose level fentanyl transdermal system may be discontinued without producing the signs and symptoms of opioid withdrawal.
2.4 Administration of Fentanyl Transdermal System
Proper handling of fentanyl transdermal system is advised in order to prevent adverse reactions, including death, associated with accidental secondary exposure to fentanyl transdermal system [ see Warnings and Precautions (5.3) ].
Application and Handling Instructions
- Patients should apply fentanyl transdermal system to intact, non‑irritated, and non-irradiated skin on a flat surface such as the chest, back, flank, or upper arm. In young children and persons with cognitive impairment, adhesion should be monitored and the upper back is the preferred location to minimize the potential of inappropriate patch removal. Hair at the application site may be clipped (not shaved) prior to system application. If the site of fentanyl transdermal system application must be cleansed prior to application of the patch, do so with clear water. Do not use soaps, oils, lotions, alcohol, or any other agents that might irritate the skin or alter its characteristics. Allow the skin to dry completely prior to patch application.
- Patients should apply fentanyl transdermal system immediately upon removal from the sealed package. The patch must not be altered (e.g., cut) in any way prior to application. Fentanyl transdermal system should not be used if the blister seal is broken or if the patch is cut or damaged.
- The transdermal system is pressed firmly in place with the palm of the hand for 30 seconds, making sure the contact is complete, especially around the edges.
- Each fentanyl transdermal system patch may be worn continuously for 72 hours. The next patch is applied to a different skin site after removal of the previous transdermal system.
- If problems with adhesion of the fentanyl transdermal system patch occur, the edges of the patch may be taped with first aid tape. If problems with adhesion persist, the patch may be overlayed with a transparent adhesive film dressing.
- If the patch falls off before 72 hours, dispose of it by folding in half and flushing down the toilet. A new patch may be applied to a different skin site.
- Patients (or caregivers who apply fentanyl transdermal system) should wash their hands immediately with soap and water after applying fentanyl transdermal system.
- Contact with unwashed or unclothed application sites can result in secondary exposure to fentanyl transdermal system and should be avoided. Examples of accidental exposure include transfer of a fentanyl transdermal system patch from an adult’s body to a child while hugging, sharing the same bed as the patient, accidental sitting on a patch and possible accidental exposure of a caregiver’s skin to the medication in the patch while applying or removing the patch.
- Instruct patients, family members, and caregivers to keep patches in a secure location out of the reach of children and of others for whom fentanyl transdermal system was not prescribed.
Avoidance of Heat
Instruct patients to avoid exposing the fentanyl transdermal system application site and surrounding area to direct external heat sources, such as heating pads or electric blankets, heat or tanning lamps, sunbathing, hot baths, saunas, hot tubs, and heated water beds, while wearing the system [see Warnings and Precautions (5.9)].
2.5 Disposal Instructions
Proper disposal of fentanyl transdermal system is advised in order to prevent adverse reactions, including death, associated with accidental secondary exposure to fentanyl transdermal system [see Warnings and Precautions (5.3)].
Patients should dispose of used patches by folding the adhesive side of the patch to itself, then flush the patch down the toilet immediately upon removal.
Patients should dispose of any patches remaining from a prescription as soon as they are no longer needed. Unused patches should be removed from their blisters, fold so that the adhesive side of the patch adheres to itself, and flush down the toilet.
3 DOSAGE FORMS AND STRENGTHS
Fentanyl transdermal system is available as:
- Fentanyl Transdermal System 25 mcg/hour (system size 10.7 cm2) is pink in color.
- Fentanyl Transdermal System 50 mcg/hour (system size 21.4 cm2) is green in color.
- Fentanyl Transdermal System 75 mcg/hour (system size 32.1 cm2) is blue in color.
- Fentanyl Transdermal System 100 mcg/hour (system size 42.8 cm2) is gray in color.
4 CONTRAINDICATIONS
Fentanyl transdermal system is contraindicated in the following patients and situations due to the risk of fatal respiratory depression:
- in patients who are not opioid‑tolerant [see Warnings and Precautions (5.2)].
- in the management of acute or intermittent pain, or in patients who require opioid analgesia for a short period of time [see Warnings and Precautions (5.2)].
- in the management of post-operative pain, including use after out-patient or day surgeries, (e.g., tonsillectomies) [see Warnings and Precautions (5.2)].
- in the management of mild pain [see Warnings and Precautions (5.2)].
- in patients with significant respiratory compromise, especially if adequate monitoring and resuscitative equipment are not readily available [see Warnings and Precautions (5.2)].
- in patients who have acute or severe bronchial asthma [see Warnings and Precautions (5.2)].
Fentanyl transdermal system is also contraindicated:
- in patients who have or are suspected of having paralytic ileus
- in patients with known hypersensitivity to fentanyl or any components of the transdermal system. Severe hypersensitivity reactions, including anaphylaxis have been observed with fentanyl transdermal system [see Adverse Reactions (6.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Abuse Potential
Fentanyl transdermal system contains fentanyl, an opioid agonist and a Schedule II controlled substance with an abuse liability similar to other opioid analgesics. Schedule II opioid substances which include hydromorphone, morphine, oxycodone, fentanyl, oxymorphone and methadone have the highest potential for abuse and risk of fatal overdose due to respiratory depression. Fentanyl transdermal system can be abused in a manner similar to other opioid agonists, legal or illicit. These risks should be considered when administering, prescribing, or dispensing fentanyl transdermal system in situations where the healthcare professional is concerned about increased risk of misuse, abuse, or diversion [see Drug Abuse and Dependence (9)].
Assess patients for their clinical risks for opioid abuse or addiction prior to being prescribed opioids. Routinely monitor all patients receiving opioids for signs of misuse, abuse and addiction since use of opioid analgesic products carries the risk of addiction even under appropriate medical use. Persons at increased risk for opioid abuse include those with a personal or family history of substance abuse (including drug or alcohol abuse or addiction) or mental illness (e.g., major depression). Patients at increased risk may still be appropriately treated with modified-release opioid formulations; however these patients will require intensive monitoring for signs of misuse, abuse, or addiction. Concerns about abuse, addiction, and diversion should not prevent the proper management of pain.
Contact local state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
5.2 Respiratory Depression and Death
Respiratory depression is the chief hazard of fentanyl transdermal system. Respiratory depression, if not immediately recognized and treated, may lead to respiratory arrest and death. Fentanyl transdermal system has a narrow indication and should be prescribed only by healthcare professionals who are knowledgeable in the administration of potent opioids and management of chronic pain [see Indications and Usage (1)]. Fentanyl transdermal system is contraindicated for use in conditions in which the risk of life-threatening respiratory depression is significantly increased, including use as an as-needed analgesic, use in non-opioid tolerant patients, acute pain, and postoperative pain [see Contraindications (4)]. Proper dosing and titration of fentanyl transdermal system are essential [see Dosage and Administration (2.3)]. Overestimating the fentanyl transdermal system dose when converting patients from another opioid medication, can result in fatal overdose with the first dose. However, respiratory depression has also been reported with use of fentanyl transdermal system in patients who are opioid-tolerant, even when fentanyl transdermal system has been used as recommended and not misused or abused.
The mean half-life of fentanyl when delivered by fentanyl transdermal system is approximately 20 to 27 hours. Serum fentanyl concentrations continue to rise for the first two system applications. In addition, significant amounts of fentanyl continue to be absorbed from the skin for 24 hours or more after the patch is removed [see Clinical Pharmacology (12.3)].
Respiratory depression from opioids is manifested by a reduced urge to breathe and a decreased rate of respiration, often associated with a “sighing” pattern of breathing (deep breaths separated by abnormally long pauses). Carbon dioxide retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids.
While serious, life-threatening or fatal respiratory depression can occur at any time during the use of fentanyl transdermal system, the potential for serious, life threatening, or fatal respiratory depression is greatest during the first two applications following initiation of dosing, or following an increase in dosage. Closely monitor patients for respiratory depression when initiating therapy with fentanyl transdermal system, especially within the initial 24 to 72 hours when serum concentrations from the initial patch will peak, and following increases in dosage. Because significant amounts of fentanyl continue to be absorbed from the skin for 24 hours or more after the patch is removed, respiratory depression may persist beyond the removal of fentanyl transdermal system. Monitor patients for respiratory depression after patch removal to ensure that the patient’s respiration has stabilized for at least 24 to 72 hours or longer as clinical symptoms dictate.
Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient’s clinical status [see Overdose (10.2)].
5.3 Accidental Exposure
A considerable amount of active fentanyl remains in fentanyl transdermal system even after use as directed. Death and other serious medical problems have occurred when children and adults were accidentally exposed to fentanyl transdermal system. Accidental or deliberate application or ingestion by a child or adolescent will cause respiratory depression that could result in death. Placing fentanyl transdermal system in the mouth, chewing it, swallowing it, or using it in ways other than indicated may cause choking or overdose that could result in death.
Advise patients about strict adherence to the recommended handling and disposal instructions in order to prevent accidental exposure to fentanyl transdermal system [see Dosage and Administration (2.4) (2.5)].
5.4 Elderly, Cachectic, and Debilitated Patients
Respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients as they may have altered pharmacokinetics due to poor fat stores, muscle wasting, or altered clearance. Therefore, monitor these patients closely, particularly when initiating therapy with fentanyl transdermal system and when given in conjunction with other drugs that depress respiration [see Warnings and Precautions (5.2) and Use in Specific Populations (8.5)] .
5.5 Chronic Pulmonary Disease
Monitor patients with significant chronic obstructive pulmonary disease or cor pulmonale, and patients having a substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression for respiratory depression, particularly when initiating therapy with fentanyl transdermal system, as in these patients, even usual therapeutic doses of fentanyl transdermal system may decrease respiratory drive to the point of apnea [see Warnings and Precautions (5.2)]. Consider the use of alternative non-opioid analgesics in these patients if possible.
5.6 Head Injuries and Increased Intracranial Pressure
Avoid use of fentanyl transdermal system in patients who may be particularly susceptible to the intracranial effects of CO2 retention such as those with evidence of increased intracranial pressure, impaired consciousness, or coma [see Warnings and Precautions (5.2)] . In addition, opioids may obscure the clinical course of patients with head injury. Monitor patients with brain tumors who may be susceptible to the intracranial effects of CO2 retention for signs of sedation and respiratory depression, particularly when initiating therapy with fentanyl transdermal system, as fentanyl transdermal system may reduce respiratory drive and CO2 retention can further increase intracranial pressure.
5.7 Interactions with Other CNS Depressants, Alcohol, and Drugs of Abuse
The concomitant use of fentanyl transdermal system with other central nervous system depressants, including, but not limited to, other opioids, sedatives, hypnotics, tranquilizers (e.g., benzodiazepines), general anesthetics, phenothiazines, skeletal muscle relaxants, and alcohol, may cause respiratory depression, hypotension, and profound sedation or coma. Monitor patients prescribed concomitant CNS active drugs for signs of sedation and respiratory depression, particularly when initiating therapy with fentanyl transdermal system, and reduce the dose of one or both agents [see Warnings and Precautions (5.2)].
5.8 Interactions with CYP3A4 Inhibitors
The concomitant use of fentanyl transdermal system with a CYP3A4 inhibitors (such as ritonavir, ketoconazole, itraconazole, troleandomycin, clarithromycin, nelfinavir, nefazadone, amiodarone, amprenavir, aprepitant, diltiazem, erythromycin, fluconazole, fosamprenavir, verapamil) may result in an increase in fentanyl plasma concentrations, which could increase or prolong adverse drug effects and may cause potentially fatal respiratory depression. Carefully monitor patients receiving fentanyl transdermal system and any CYP3A4 inhibitor for signs of sedation and respiratory depression for an extended period of time, and make dosage adjustments if warranted [see Warnings and Precautions (5.2), Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
5.9 Application of External Heat
Exposure to heat may increase fentanyl absorption and there have been reports of overdose and death as a result of exposure to heat. A clinical pharmacology study conducted in healthy adult subjects has shown that the application of heat over the fentanyl transdermal system increased fentanyl exposure [see Clinical Pharmacology (12.3)].Warn patients to avoid exposing the fentanyl transdermal system application site and surrounding area to direct external heat sources [see Dosage and Administration (2.4)].
5.10 Patients with Fever
Based on a pharmacokinetic model, serum fentanyl concentrations could theoretically increase by approximately one-third for patients with a body temperature of 40°C (104°F) due to temperature-dependent increases in fentanyl released from the system and increased skin permeability. Monitor patients wearing fentanyl transdermal system who develop fever closely for opioid side effects and reduce the fentanyl transdermal system dose if necessary. Warn patients to avoid strenuous exertion that leads to increased core body temperature while wearing fentanyl transdermal system to avoid the risk of potential overdose and death.
5.11 Cardiac Disease
Fentanyl transdermal system may produce bradycardia. Monitor patients with bradyarrhythmias closely for changes in heart rate, particularly when initiating therapy with fentanyl transdermal system.
5.12 Hepatic Impairment
A clinical pharmacology study with fentanyl transdermal system in patients with cirrhosis has shown that systemic fentanyl exposure increased in these patients. Because of the long half-life of fentanyl when administered as fentanyl transdermal system and hepatic metabolism of fentanyl, avoid use of fentanyl transdermal system in patients with severe hepatic impairment. Insufficient information exists to make precise dosing recommendations regarding the use of fentanyl transdermal system in patients with impaired hepatic function. Therefore, to avoid starting patients with mild to moderate hepatic impairment on too high of a dose, start with one half of the usual dosage of fentanyl transdermal system. Closely monitor for signs of sedation and respiratory depression, including at each dosage increase [see Dosing and Administration (2.2), Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
5.13 Renal Impairment
A clinical pharmacology study with intravenous fentanyl in patients undergoing kidney transplantation has shown that patients with high blood urea nitrogen level had low fentanyl clearance. Because of the long half-life of fentanyl when administered as fentanyl transdermal system, avoid the use of fentanyl transdermal system in patients with severe renal impairment. Insufficient information exists to make precise dosing recommendations regarding the use of fentanyl transdermal system in patients with impaired renal function. Therefore, to avoid starting patients with mild to moderate renal impairment on too high of a dose, start with one half of the usual dosage of fentanyl transdermal system. Closely monitor for signs of sedation and respiratory depression, including at each dosage increase [see Dosing and Administration (2.2), Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
5.14 Use in Pancreatic/Biliary Tract Disease
Fentanyl transdermal system may cause spasm of the sphincter of Oddi. Monitor patients with biliary tract disease, including acute pancreatitis for worsened symptoms. Fentanyl transdermal system may cause increases in the serum amylase concentration.
5.15 Avoidance of Withdrawal
Opioid withdrawal symptoms (such as nausea, vomiting, diarrhea, anxiety, and shivering) are possible in some patients after conversion to another opioid or when decreasing or discontinuing fentanyl transdermal system. Gradual reduction of the dose of fentanyl transdermal system is recommended [see Dosage and Administration (2.3) and Drug Abuse and Dependence (9)].
5.16 Driving and Operating Machinery
Strong opioid analgesics impair the mental or physical abilities required for the performance of potentially dangerous tasks, such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of the fentanyl transdermal system.
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Abuse Potential [see Warnings and Precautions (5.1)]
- Respiratory Depression [see Warnings and Precautions (5.2)]
- Accidental Exposure [see Warnings and Precautions (5.3)]
- Elderly, Cachectic, and Debilitated Patients [see Warnings and Precautions (5.4)]
- Chronic Pulmonary Disease [see Warnings and Precautions (5.5)]
- Head Injuries and Increased Intracranial Pressure [see Warnings and Precautions (5.6)]
- Interactions with Other CNS Depressants, Alcohol, and Drugs of Abuse [see Warnings and Precautions (5.7)]
- Interactions with CYP3A4 Inhibitors [see Warnings and Precautions (5.8)]
- Application of External Heat [see Warnings and Precautions (5.9)]
- Patients with Fever [see Warnings and Precautions (5.10)]
- Cardiac Disease [see Warnings and Precautions (5.11)]
- Hepatic Impairment [see Warnings and Precautions (5.12)]
- Renal Impairment [see Warnings and Precautions (5.13)]
- Use in Pancreatic/Biliary Tract Disease [see Warnings and Precautions (5.14)]
- Avoidance of Withdrawal [see Warnings and Precautions (5.15)]
- Driving and Operating Machinery [see Warnings and Precautions (5.16)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
6.1 Clinical Trial Experience
The safety of fentanyl transdermal systemwas evaluated in 216 patients who took at least one dose of fentanyl transdermal system in a multicenter, double-blind, randomized, placebo-controlled clinical trial of fentanyl transdermal system. This trial examined patients over 40 years of age with severe pain induced by osteoarthritis of the hip or knee and who were in need of and waiting for joint replacement.
The most common adverse reactions (≥5%) in a double-blind, randomized, placebo-controlled clinical trial in patients with severe pain were nausea, vomiting, somnolence, dizziness, insomnia, constipation, hyperhidrosis, fatigue, feeling cold, and anorexia. Other common adverse reactions (≥5%) reported in clinical trials in patients with chronic malignant or nonmalignant pain were headache and diarrhea. Adverse reactions reported for ≥1% of fentanyl transdermal system-treated patients and with an incidence greater than placebo-treated patients are shown in Table 3.
The most common adverse reactions that were associated with discontinuation in patients with pain (causing discontinuation in ≥1% of patients) were depression, dizziness, somnolence, headache, nausea, vomiting, constipation, hyperhidrosis, and fatigue.
Adverse reactions not reported in Table 1 that were reported by ≥1% of fentanyl transdermal system‑treated adult and pediatric patients (N=1854) in 11 controlled and uncontrolled clinical trials of fentanyl transdermal system used for the treatment of chronic malignant or nonmalignant pain are shown in Table 4.
| Table 4. Adverse Reactions Reported by ≥1% of Fentanyl Transdermal System-treated Patients in 11 Clinical Trials of Fentanyl Transdermal System | |
| System/Organ ClassAdverse Reaction | Fentanyl Transdermal System% (N=1854) |
| Gastrointestinal disorders | |
| Diarrhea | 10 |
| Abdominal pain | 3 |
| Immune system disorders | |
| Hypersensitivity | 1 |
| Nervous system disorders | |
| Headache | 12 |
| Tremor | 3 |
| Paresthesia | 2 |
| Psychiatric disorders | |
| Anxiety | 3 |
| Confusional state | 2 |
| Hallucination | 1 |
| Renal and urinary disorders | |
| Urinary retention | 1 |
| Skin and subcutaneous tissue disorders | |
| Erythema | 1 |
The following adverse reactions occurred in adult and pediatric patients with an overall frequency of <1% and are listed in descending frequency within each System/Organ Class:
Cardiac disorders: cyanosis
Eye disorders: miosis
Gastrointestinal disorders: subileus
General disorders and administration site conditions: application site reaction, influenza-like illness, application site hypersensitivity, drug withdrawal syndrome, application site dermatitis
Musculoskeletal and connective tissue disorders: muscle twitching
Nervous system disorders: hypoesthesia
Psychiatric disorders: disorientation, euphoric mood
Reproductive system and breast disorders: erectile dysfunction, sexual dysfunction
Respiratory, thoracic and mediastinal disorders: respiratory depression
Skin and subcutaneous tissue disorders: eczema, dermatitis allergic, dermatitis contact
Pediatrics
The safety of fentanyl transdermal system was evaluated in three open-label trials in 289 pediatric patients with chronic pain, 2 years of age through 18 years of age. Adverse reactions reported by ≥1% of fentanyl transdermal system-treated pediatric patients are shown in Table 5.
| Table 5. Adverse Reactions Reported by ≥1% of Fentanyl Transdermal System-treated Pediatric Patients in 3 Clinical Trials of Fentanyl Transdermal System | |
| System/Organ Class Adverse Reaction | Fentanyl Transdermal System% (N=289) |
| Gastrointestinal disorders | |
| Vomiting | 34 |
| Nausea | 24 |
| Constipation | 13 |
| Diarrhea | 13 |
| Abdominal pain | 9 |
| Abdominal pain upper | 4 |
| Dry mouth | 2 |
| General disorders and administration site conditions | |
| Edema peripheral | 5 |
| Fatigue | 2 |
| Application site reaction | 1 |
| Asthenia | 1 |
| Immune system disorders | |
| Hypersensitivity | 3 |
| Metabolism and nutrition disorders | |
| Anorexia | 4 |
| Musculoskeletal and connective tissue disorders | |
| Muscle spasms | 2 |
| Nervous system disorders | |
| Headache | 16 |
| Somnolence | 5 |
| Dizziness | 2 |
| Tremor | 2 |
| Hypoesthesia | 1 |
| Psychiatric disorders | |
| Insomnia | 6 |
| Anxiety | 4 |
| Depression | 2 |
| Hallucination | 2 |
| Renal and urinary disorders | |
| Urinary retention | 3 |
| Respiratory, thoracic and mediastinal disorders | |
| Respiratory depression | 1 |
| Skin and subcutaneous tissue disorders | |
| Pruritus | 13 |
| Rash | 6 |
| Hyperhidrosis | 3 |
| Erythema | 3 |
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of fentanyl transdermal system. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency.
Cardiac disorders: tachycardia, bradycardia
Eye disorders: vision blurred
Gastrointestinal disorders: ileus, dyspepsia
General disorders and administration site conditions: feeling of body temperature change
Immune system disorders: anaphylactic shock, anaphylactic reaction, anaphylactoid reaction
Investigations: weight decreased
Nervous system disorders: convulsions (including clonic convulsions and grand mal convulsion), amnesia
Psychiatric disorders: agitation
Respiratory, thoracic, and mediastinal disorders: respiratory distress, apnea, bradypnea, hypoventilation, dyspnea
Vascular disorders: hypotension, hypertension
7 DRUG INTERACTIONS
7.1 Agents Affecting Cytochrome P450 3A4 Isoenzyme System
Fentanyl is metabolized mainly via the human cytochrome P450 3A4 isoenzyme system (CYP3A4). Coadministration with agents that induce CYP3A4 activity may reduce the efficacy of fentanyl transdermal system. The concomitant use of fentanyl transdermal system with a CYP3A4 inhibitor (such as ritonavir, ketoconazole, itraconazole, troleandomycin, clarithromycin, nelfanivir, nefazadone, amiodarone, amprenavir, aprepitant, diltiazem, erythromycin, fluconazole, fosamprenavir, verapamil, or grapefruit juice) may result in an increase in fentanyl plasma concentrations, which could increase or prolong adverse drug effects and may cause fatal respiratory depression. Closely monitor patients receiving fentanyl transdermal system and any CYP3A4 inhibitor and reduce the dosage of fentanyl transdermal system if warranted [see Clinical Pharmacology (12.3)].
7.2 Central Nervous System Depressants
The concomitant use of fentanyl transdermal system with other central nervous system depressants, including but not limited to other opioids, sedatives, hypnotics, tranquilizers (e.g., benzodiazepines), general anesthetics, phenothiazines, skeletal muscle relaxants, and alcohol, may cause respiratory depression, hypotension, and profound sedation, or potentially result in coma or death. Monitor patients closely when central nervous system depressants are used concomitantly with fentanyl transdermal system and reduce the dose of one or both agents.
7.3 MAO Inhibitors
Avoid use of fentanyl transdermal system in the patient who would require the concomitant administration of a monoamine oxidase (MAO) inhibitor, or within 14 days of stopping such treatment because severe and unpredictable potentiation by MAO inhibitors has been reported with opioid analgesics.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy C: There are no adequate and well-controlled studies in pregnant women. Fentanyl transdermal system should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
The potential effects of fentanyl on embryo-fetal development were studied in the rat, mouse, and rabbit models. Published literature reports that administration of fentanyl (0, 10, 100, or 500 mcg/kg/day) to pregnant female Sprague-Dawley rats from day 7 to 21 via implanted microosmotic minipumps did not produce any evidence of teratogenicity (the high dose is approximately 2 times the daily human dose administered by a 100 mcg/h patch on a mg/m2 basis). In contrast, the intravenous administration of fentanyl (0, 0.01, or 0.03 mg/kg) to bred female rats from gestation day 6 to 18 suggested evidence of embryotoxicity and a slight increase in mean delivery time in the 0.03 mg/kg/day group. There was no clear evidence of teratogenicity noted.
Pregnant female New Zealand White rabbits were treated with fentanyl (0, 0.025, 0.1, 0.4 mg/kg) via intravenous infusion from day 6 to day 18 of pregnancy. Fentanyl produced a slight decrease in the body weight of the live fetuses at the high dose, which may be attributed to maternal toxicity. Under the conditions of the assay, there was no evidence for fentanyl induced adverse effects on embryo-fetal development at doses up to 0.4 mg/kg (approximately 3 times the daily human dose administered by a 100 mcg/h patch on a mg/m2 basis).
Chronic maternal treatment with fentanyl during pregnancy has been associated with transient respiratory depression, behavioral changes, or seizures characteristic of neonatal abstinence syndrome in newborn infants. Symptoms of neonatal respiratory or neurological depression were no more frequent than expected in most studies of infants born to women treated acutely during labor with intravenous or epidural fentanyl. Transient neonatal muscular rigidity has been observed in infants whose mothers were treated with intravenous fentanyl.
The potential effects of fentanyl on prenatal and postnatal development were examined in the rat model. Female Wistar rats were treated with 0, 0.025, 0.1, or 0.4 mg/kg/day fentanyl via intravenous infusion from day 6 of pregnancy through 3 weeks of lactation. Fentanyl treatment (0.4 mg/kg/day) significantly decreased body weight in male and female pups and also decreased survival in pups at day 4. Both the mid-dose and high-dose of fentanyl animals demonstrated alterations in some physical landmarks of development (delayed incisor eruption and eye opening) and transient behavioral development (decreased locomotor activity at day 28 which recovered by day 50). The mid-dose and the high-dose are 0.4 and 1.6 times the daily human dose administered by a 100 mcg/h patch on a mg/m2 basis.
8.2 Labor and Delivery
Fentanyl readily passes across the placenta to the fetus; therefore, fentanyl transdermal system is not recommended for analgesia during labor and delivery.
8.3 Nursing Mothers
Fentanyl is excreted in human milk; therefore, fentanyl transdermal system is not recommended for use in nursing women because of the possibility of effects in their infants.
8.4 Pediatric Use
The safety of fentanyl transdermal system was evaluated in three open-label trials in 289 pediatric patients with chronic pain, 2 years of age through 18 years of age. Starting doses of 25 mcg/h and higher were used by 181 patients who had been on prior daily opioid doses of at least 45 mg/day of oral morphine or an equianalgesic dose of another opioid. Initiation of fentanyl transdermal system therapy in pediatric patients taking less than 60 mg/day of oral morphine or an equianalgesic dose of another opioid has not been evaluated in controlled clinical trials.
The safety and effectiveness of fentanyl transdermal system in children under 2 years of age have not been established.
To guard against excessive exposure to fentanyl transdermal system by young children, advise caregivers to strictly adhere to recommended fentanyl transdermal system application and disposal instructions [see Dosage and Administration (2.4) (2.5) and Warnings and Precautions (5.3)].
8.5 Geriatric Use
Clinical studies of fentanyl transdermal system did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Data from intravenous studies with fentanyl suggest that the elderly patients may have reduced clearance and a prolonged half-life. Moreover, elderly patients may be more sensitive to the active substance than younger patients. A study conducted with the fentanyl transdermal system patch in elderly patients demonstrated that fentanyl pharmacokinetics did not differ significantly from young adult subjects, although peak serum concentrations tended to be lower and mean half-life values were prolonged to approximately 34 hours [see Clinical Pharmacology (12.3)].
Monitor geriatric patients closely for signs of sedation and respiratory depression, particularly when initiating therapy with fentanyl transdermal system and when given in conjunction with other drugs that depress respiration [see Warnings and Precautions (5.2) (5.4)].
8.6 Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of fentanyl transdermal system has not been fully evaluated. A clinical pharmacology study with fentanyl transdermal system in patients with cirrhosis has shown that systemic fentanyl exposure increased in these patients. Because there is in vitro and in vivo evidence of extensive hepatic contribution to the elimination of fentanyl transdermal system, hepatic impairment would be expected to have significant effects on the pharmacokinetics of fentanyl transdermal system. Avoid use of fentanyl transdermal system in patients with severe hepatic impairment [see Dosing and Administration (2.2), Warnings and Precautions (5.12) and Clinical Pharmacology 12.3)].
8.7 Renal Impairment
The effect of renal impairment on the pharmacokinetics of fentanyl transdermal system has not been fully evaluated. A clinical pharmacology study with intravenous fentanyl in patients undergoing kidney transplantation has shown that patients with high blood urea nitrogen level had low fentanyl clearance. Because there is in vivo evidence of renal contribution to the elimination of fentanyl transdermal system, renal impairment would be expected to have significant effects on the pharmacokinetics of fentanyl transdermal system. Avoid the use of fentanyl transdermal system in patients with severe renal impairment [see Dosing and Administration (2.2), Warnings and Precautions (5.13) and Clinical Pharmacology (12.3)].
8.8 Neonatal Opioid Withdrawal Syndrome
Chronic maternal use of fentanyl can affect the neonate with subsequent withdrawal signs. Neonatal withdrawal syndrome presents as irritability, hyperactivity, and abnormal sleep pattern, high pitched cry, tremor, vomiting, diarrhea, and failure to gain weight. The onset, duration and severity of neonatal withdrawal syndrome vary based on the drug used, duration of use, the dosage of last maternal use, and rate of elimination of the drug by the newborn. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening and should be treated according to protocols developed by neonatology experts.
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Fentanyl transdermal system contains fentanyl, a potent Schedule II opioid agonist. Schedule II opioid substances, which include hydromorphone, methadone, morphine, oxycodone, and oxymorphone, have the highest potential for abuse and risk of fatal overdose due to respiratory depression. Fentanyl transdermal system can be abused and is subject to criminal diversion [see Warnings and Precautions (5.1)] .
9.2 Abuse
Addiction is a primary, chronic, neurobiologic disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common.
“Drug seeking” behavior is very common in addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated “loss” of prescriptions, tampering with prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). “Doctor shopping” to obtain additional prescriptions is common among drug abusers and people suffering from untreated addiction.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physicians should be aware that addiction may be accompanied by concurrent tolerance and symptoms of physical dependence. In addition, abuse of opioids can occur in the absence of true addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. Since fentanyl transdermal system may be diverted for non-medical use,careful recordkeeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic reevaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
9.3 Dependence
Tolerance is a state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time. Tolerance may occur to both the desired and undesired effects of drugs, and may develop at different rates for different effects.Physical dependence is a state of adaptation that is manifested by an opioid specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood concentration of the drug, and/or administration of an antagonist. The opioid abstinence or withdrawal syndrome is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, piloerection, myalgia, mydriasis, irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate. In general, opioids should not be abruptly discontinued [see Dosage and Administration (2.3)].
10 OVERDOSAGE
10.1 Clinical Presentation
Acute overdosage with opioids can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and sometimes bradycardia, hypotension and death. The pharmacokinetic characteristics of fentanyl transdermal system must also be taken into account when treating the overdose. Even in the face of improvement, continued medical monitoring is required because of the possibility of extended effects. Deaths due to overdose have been reported with abuse and misuse of fentanyl transdermal system.
10.2 Treatment
Give primary attention to the reestablishment of a patent airway and institution of assisted or controlled ventilation. Employ supportive measures (including oxygen and vasopressors) in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias will require advanced life support techniques. Remove all fentanyl transdermal systems.
The pure opioid antagonists, such as naloxone, are specific antidotes to respiratory depression from opioid overdose. Since the duration of reversal is expected to be less than the duration of action of fentanyl, carefully monitor the patient until spontaneous respiration is reliably reestablished. After fentanyl transdermal system removal, serum fentanyl concentrations decline gradually, falling about 50% in approximately 20 to 27 hours. Therefore, management of an overdose must be monitored accordingly, at least 72 to 96 hours beyond the overdose.
Only administer opioid antagonists in the presence of clinically significant respiratory or circulatory depression secondary to hydromorphone overdose. In patients who are physically dependent on any opioid agonist including fentanyl transdermal system, an abrupt or complete reversal of opioid effects may precipitate an acute abstinence syndrome. The severity of the withdrawal syndrome produced will depend on the degree of physical dependence and the dose of the antagonist administered. Please see the prescribing information for the specific opioid antagonist for details of their proper use.
11 DESCRIPTION
Fentanyl transdermal system is a transdermal system containing fentanyl. The chemical name is N‑phenyl-N-(1-(2-phenylethyl)-4-piperidinyl) propanamide. The structural formula is:

The molecular weight of fentanyl base is 336.5, and the empirical formula is C22H28N2O. The n‑octanol: water partition coefficient is 860:1. The pKa is 8.4.
System Components and Structure
The amount of fentanyl released from each system per hour is proportional to the surface area (25 mcg/h per 10.7 cm2). The composition per unit area of all system sizes is identical.
| Dose |
Size(cm2) | Fentanyl Content(mg) | Color of Printing on Back of Patch |
| 25 | 10.7 | 2.76 | Pink |
| 50 | 21.4 | 5.52 | Green |
| 75 | 32.1 | 8.28 | Blue |
| 100 | 42.8 | 11.04 | Gray |
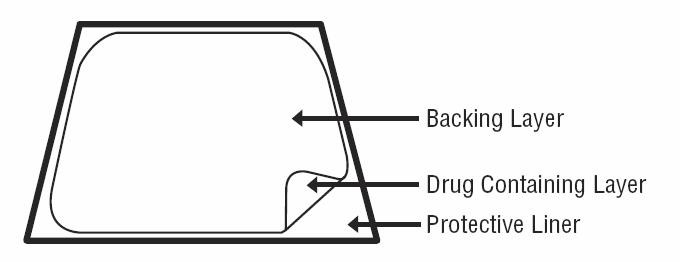
Fentanyl transdermal system is a rectangular transparent unit comprising a protective liner and two functional layers. Proceeding from the outer surface toward the surface adhering to skin, these layers are:
- a backing layer of polyethylene/aluminum/polyester film; 2) a drug-in-adhesive layer. Before use, a protective liner covering the adhesive layer is removed and discarded.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fentanyl is an opioid analgesic. Fentanyl interacts predominately with the opioid mu‑receptor. These mu-binding sites are distributed in the human brain, spinal cord, and other tissues.
12.2 Pharmacodynamics
Central Nervous System Effects
Fentanyl exerts its principal pharmacologic effects on the central nervous system. Central nervous system effects increase with increasing serum fentanyl concentrations.
In addition to analgesia, alterations in mood, euphoria, dysphoria, and drowsiness commonly occur. Fentanyl depresses the respiratory centers, depresses the cough reflex, and constricts the pupils. Analgesic blood concentrations of fentanyl may cause nausea and vomiting directly by stimulating the chemoreceptor trigger zone, but nausea and vomiting are significantly more common in ambulatory than in recumbent patients, as is postural syncope.
Ventilatory Effects
In clinical trials of 357 non-opioid tolerant subjects treated with fentanyl transdermal system, 13 subjects experienced hypoventilation. Hypoventilation was manifested by respiratory rates of less than 8 breaths/minute or a pCO2 greater than 55 mm Hg. In these studies, the incidence of hypoventilation was higher in nontolerant women (10) than in men (3) and in subjects weighing less than 63 kg (9 of 13). Although subjects with prior impaired respiration were not common in the trials, they had higher rates of hypoventilation. In addition, post-marketing reports have been received that describe opioid-naive post-operative patients who have experienced clinically significant hypoventilation and death with fentanyl transdermal system.
Hypoventilation can occur throughout the therapeutic range of fentanyl serum concentrations, especially for patients who have an underlying pulmonary condition or who receive concomitant opioids or other CNS drugs associated with hypoventilation. The use of fentanyl transdermal system is contraindicated in patients who are not tolerant to opioid therapy.
Gastrointestinal Tract and Other Smooth Muscle
Opioids increase the tone and decrease the propulsive contractions of the smooth muscle of the gastrointestinal tract. The resultant prolongation in gastrointestinal transit time may be responsible for the constipating effect of fentanyl. Because opioids may increase biliary tract pressure, some patients with biliary colic may experience worsening rather than relief of pain.
While opioids generally increase the tone of urinary tract smooth muscle, the net effect tends to be variable, in some cases producing urinary urgency, in others, difficulty in urination.
Cardiovascular Effects
Fentanyl may cause orthostatic hypotension and fainting. Fentanyl may infrequently produce bradycardia. The incidence of bradycardia in clinical trials with fentanyl transdermal system was less than 1%.
Histamine assays and skin wheal testing in clinical studies indicate that clinically significant histamine release rarely occurs with fentanyl administration. Clinical assays show no clinically significant histamine release in dosages up to 50 mcg/kg.
12.3 Pharmacokinetics
Absorption
Fentanyl transdermal system is a drug-in-adhesive matrix designed formulation. Fentanyl is released from the matrix at a nearly constant amount per unit time. The concentration gradient existing between the matrix and the lower concentration in the skin drives drug release. Fentanyl moves in the direction of the lower concentration at a rate determined by the matrix and the diffusion of fentanyl through the skin layers. While the actual rate of fentanyl delivery to the skin varies over the 72-hour application period, each system is labeled with a nominal flux which represents the average amount of drug delivered to the systemic circulation per hour across average skin.
While there is variation in dose delivered among patients, the nominal flux of the systems (25, 50, 75, and 100 mcg of fentanyl per hour) is sufficiently accurate as to allow individual titration of dosage for a given patient.
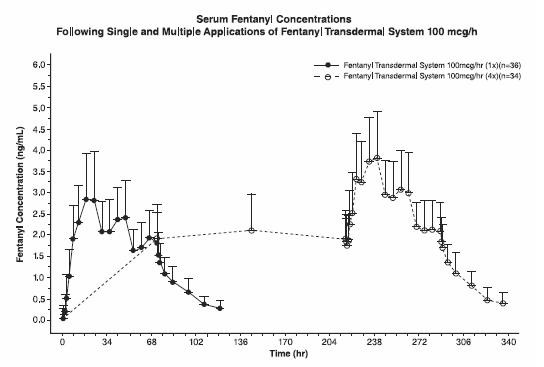
Following fentanyl transdermal system application, the skin under the system absorbs fentanyl, and a depot of fentanyl concentrates in the upper skin layers. Fentanyl then becomes available to the systemic circulation. Serum fentanyl concentrations increase gradually following initial fentanyl transdermal system application, generally leveling off between 12 and 24 hours and remaining relatively constant, with some fluctuation, for the remainder of the 72-hour application period. Peak serum concentrations of fentanyl generally occurred between 20 and 72 hours after initial application (see Table 6). Serum fentanyl concentrations achieved are proportional to the fentanyl transdermal system delivery rate. With continuous use, serum fentanyl concentrations continue to rise for the first two system applications. By the end of the second 72-hour application, a steady-state serum concentration is reached and is maintained during subsequent applications of a patch of the same size (see Figure 1). Patients reach and maintain a steady-state serum concentration that is determined by individual variation in skin permeability and body clearance of fentanyl.
After system removal, serum fentanyl concentrations decline gradually, falling about 50% in approximately 20 to 27 hours. Continued absorption of fentanyl from the skin accounts for a slower disappearance of the drug from the serum than is seen after an IV infusion, where the apparent half-life is approximately 7 (range 3 to 12) hours.
A clinical pharmacology study conducted in healthy adult subjects has shown that the application of heat over the fentanyl transdermal system increased mean overall fentanyl exposure by 120% and average maximum fentanyl level by 61%.
| TABLE 6: FENTANYL PHARMACOKINETIC PARAMETERS FOLLOWING FIRST 72-HOUR APPLICATION OF A FENTANYL TRANSDERMAL SYSTEM | ||
| Mean (SD) Time to Maximal ConcentrationTmax(h) | Mean (SD) Maximal Concentration Cmax (ng/mL) | |
| Fentanyl Transdermal System25 mcg/h | 31.7 (16.5) | 0.85 (0.26) |
| Fentanyl Transdermal System 50 mcg/h | 32.8 (15.6) | 1.72 (0.53) |
| Fentanyl Transdermal System 75 mcg/h | 35.8 (14.1) | 2.32 (0.86) |
| Fentanyl Transdermal System 100 mcg/h | 29.9 (13.3) | 3.36 (1.28) |
| NOTE: After system removal there is continued systemic absorption from residual fentanyl in the skin so that serum concentrations fall 50%, on average, in approximately 20 to 27 hours | ||
Figure 1 Serum Fentanyl ConcentrationsFollowing Single and Multiple Applications of Fentanyl Transdermal System 100 mcg/h
| TABLE 7: RANGE OF PHARMACOKINETIC PARAMETERS OF INTRAVENOUS FENTANYL IN PATIENTS | |||
| Clearance (L/h) Range[70 kg] | Volume of Distribution VSS(L/kg) Range | Half-Life t1/2 (h) Range | |
| Surgical Patients | 27 - 75 | 3 - 8 | 3 - 12 |
| Hepatically Impaired Patients | 3 - 80+ | 0.8 - 8+ | 4 - 12+ |
| Renally Impaired Patients | 30 - 78 | – | – |
| +Estimated | |||
| NOTE: Information on volume of distribution and half-life not available for renally impaired patients. | |||
Distribution
Fentanyl plasma protein binding capacity decreases with increasing ionization of the drug. Alterations in pH may affect its distribution between plasma and the central nervous system. Fentanyl accumulates in the skeletal muscle and fat and is released slowly into the blood. The average volume of distribution for fentanyl is 6 L/kg (range 3‑8; N=8).
Metabolism
Fentanyl is metabolized primarily via human cytochrome P450 3A4 isoenzyme system. In humans, the drug appears to be metabolized primarily by oxidative N‑dealkylation to norfentanyl and other inactive metabolites that do not contribute materially to the observed activity of the drug.
Excretion
Within 72 hours of IV fentanyl administration, approximately 75% of the dose is excreted in urine, mostly as metabolites with less than 10% representing unchanged drug. Approximately 9% of the dose is recovered in the feces, primarily as metabolites. Mean values for unbound fractions of fentanyl in plasma are estimated to be between 13 and 21%.
Skin does not appear to metabolize fentanyl delivered transdermally. This was determined in a human keratinocyte cell assay and in clinical studies in which 92% of the dose delivered from the system was accounted for as unchanged fentanyl that appeared in the systemic circulation.
Hepatic Impairment
Information on the effect of hepatic impairment on the pharmacokinetics of fentanyl transdermal system is limited. The pharmacokinetics of fentanyl transdermal system delivering 50 mcg/hour of fentanyl for 72 hours was evaluated in patients hospitalized for surgery. Compared to the controlled patients (n=8), Cmax and AUC in the patients with cirrhosis (n=9) increased 35% and 73%, respectively.
Because there is in vitro and in vivo evidence of extensive hepatic contribution to the elimination of fentanyl transdermal system, hepatic impairment would be expected to have significant effects on the pharmacokinetics of fentanyl transdermal system. Avoid use of fentanyl transdermal system in patients with severe hepatic impairment [see Dosing and Administration (2.2), Warnings and Precautions (5.12) and Use in Specific Populations (8.6)].
Renal Impairment
Information on the effect of renal impairment on the pharmacokinetics of fentanyl transdermal system is limited. The pharmacokinetics of intravenous injection of 25 mcg/kg fentanyl was evaluated in patients (n=8) undergoing kidney transplantation. An inverse relationship between blood urea nitrogen level and fentanyl clearance was found.
Because there is in vivo evidence of renal contribution to the elimination of fentanyl transdermal system, renal impairment would be expected to have significant effects on the pharmacokinetics of fentanyl transdermal system. Avoid the use of fentanyl transdermal system in patients with severe renal impairment [see Dosing and Administration (2.2), Warnings and Precautions (5.13) and Use in Specific Populations (8.7)].
Pediatric Use
In 1.5 to 5 year old, non-opioid-tolerant pediatric patients, the fentanyl plasma concentrations were approximately twice as high as that of adult patients. In older pediatric patients, the pharmacokinetic parameters were similar to that of adults. However, these findings have been taken into consideration in determining the dosing recommendations for opioid-tolerant pediatric patients (2 years of age and older). For pediatric dosing information, refer to [see Dosing and Administration (2.2)] .
Geriatric Use
Data from intravenous studies with fentanyl suggest that the elderly patients may have reduced clearance and a prolonged half-life. Moreover elderly patients may be more sensitive to the active substance than younger patients. A study conducted with the fentanyl transdermal system in elderly patients demonstrated that fentanyl pharmacokinetics did not differ significantly from young adult subjects, although peak serum concentrations tended to be lower and mean half-life values were prolonged to approximately 34 hours. In this study, a single fentanyl transdermal system 100 mcg/hour patch was applied to a skin site on the upper outer arm in a group of healthy elderly Caucasians ≥65 years old (n=21, mean age 71 years) and worn for 72 hours. The mean Cmax and AUC∞ values were approximately 8% lower and 7% higher, respectively, in the elderly subjects as compared with subjects 18 to 45 years old. Inter-subject variability in AUC∞ was higher in elderly subjects than in healthy adult subjects 18 to 45 years (58% and 37%, respectively). The mean half-life value was longer in subjects ≥65 years old than in subjects 18 to 45 years old (34.4 hours versus 23.5 hours) [see Warnings and Precautions (5.4) and Use in Specific Populations (8.5)].
Drug Interactions
The interaction between ritonavir, a CYP3A4 inhibitor, and fentanyl was investigated in eleven healthy volunteers in a randomized crossover study. Subjects received oral ritonavir or placebo for 3 days. The ritonavir dose was 200 mg t.i.d. on Day 1 and 300 mg t.i.d. on Day 2 followed by one morning dose of 300 mg on Day 3. On Day 2, fentanyl was given as a single IV dose at 5 mcg/kg two hours after the afternoon dose of oral ritonavir or placebo. Naloxone was administered to counteract the side effects of fentanyl. The results suggested that ritonavir might decrease the clearance of fentanyl by 67%, resulting in a 174% (range 52% to 420%) increase in fentanyl AUC0‑∞. Coadministration of ritonavir in patients receiving fentanyl transdermal system has not been studied; however, an increase in fentanyl AUC is expected [see Box Warning and Warnings and Precautions (5.7) and Drug Interactions (7.1)].
Fentanyl is metabolized mainly via the human cytochrome P450 3A4 isoenzyme system (CYP3A4), therefore, potential interactions may occur when fentanyl transdermal system is given concurrently with agents that affect CYP3A4 activity. Coadministration with agents that induce CYP3A4 activity may reduce the efficacy of fentanyl transdermal system. The concomitant use of transdermal fentanyl with all CYP3A4 inhibitors (such as ritonavir, ketoconazole, itraconazole, troleandomycin, clarithromycin, nelfinavir, nefazadone, amiodarone, amprenavir, aprepitant, diltiazem, erythromycin, fluconazole, fosamprenavir, verapamil, or grapefruit juice) may result in an increase in fentanyl plasma concentrations, which could increase or prolong adverse drug effects and may cause potentially fatal respiratory depression. Carefully monitor patients receiving fentanyl transdermal system and any CYP3A4 inhibitor for signs of respiratory depression for an extended period of time and adjust the dosage if warranted [see Box Warning and Warnings and Precautions (5.7)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenesis
In a two-year carcinogenicity study conducted in rats, fentanyl was not associated with an increased incidence of tumors at subcutaneous doses up to 33 mcg/kg/day in males or 100 mcg/kg/day in females (0.16 and 0.39 times the human daily exposure obtained via the 100 mcg/h patch based on AUC0-24h comparison).
Mutagenesis
There was no evidence of mutagenicity in the Ames Salmonella mutagenicity assay, the primary rat hepatocyte unscheduled DNA synthesis assay, the BALB/c 3T3 transformation test, and the human lymphocyte and CHO chromosomal aberration in vitro assays.
Impairment of Fertility
The potential effects of fentanyl on male and female fertility were examined in the rat model via two separate experiments. In the male fertility study, male rats were treated with fentanyl (0, 0.025, 0.1 or 0.4 mg/kg/day) via continuous intravenous infusion for 28 days prior to mating; female rats were not treated. In the female fertility study, female rats were treated with fentanyl (0, 0.025, 0.1 or 0.4 mg/kg/day) via continuous intravenous infusion for 14 days prior to mating until day 16 of pregnancy; male rats were not treated. Analysis of fertility parameters in both studies indicated that an intravenous dose of fentanyl up to 0.4 mg/kg/day to either the male or the female alone produced no effects on fertility (this dose is approximately 1.6 times the daily human dose administered by a 100 mcg/h patch on a mg/m2 basis). In a separate study, a single daily bolus dose of fentanyl was shown to impair fertility in rats when given in intravenous doses of 0.3 times the human dose for a period of 12 days.
14 CLINICAL STUDIES
Fentanyl transdermal system as therapy for pain due to cancer has been studied in 153 patients. In this patient population, fentanyl transdermal system has been administered in doses of 25 mcg/h to 600 mcg/h. Individual patients have used fentanyl transdermal system continuously for up to 866 days. At one month after initiation of fentanyl transdermal system therapy, patients generally reported lower pain intensity scores as compared to a prestudy analgesic regimen of oral morphine.
The duration of fentanyl transdermal system use varied in cancer patients; 56% of patients used fentanyl transdermal system for over 30 days, 28% continued treatment for more than 4 months, and 10% used fentanyl transdermal system for more than 1 year.
In the pediatric population, the safety of fentanyl transdermal system has been evaluated in 289 patients with chronic pain 2 to 18 years of age. The duration of fentanyl transdermal system use varied; 20% of pediatric patients were treated for ≤ 15 days; 46% for 16 to 30 days; 16% for 31 to 60 days; and 17% for at least 61 days. Twenty-five patients were treated with fentanyl transdermal system for at least 4 months and 9 patients for more than 9 months.
16 HOW SUPPLIED/STORAGE AND HANDLING
Fentanyl transdermal system is supplied in cartons containing 5 individually packaged systems. See chart for information regarding individual systems.
| Fentanyl Transdermal System Dose(mcg/h) | System Size(cm2) | Fentanyl Content(mg) | Color of Printing on Back of Patch | NDCNumber for Blister Containing Individual System | NDCNumber for Carton Containing 5 Systems |
| Fentanyl Transdermal System-25 | 10.7 | 2.76 | Pink | 60505-7006-0 | 60505-7006-2 |
| Fentanyl Transdermal System-50 | 21.4 | 5.52 | Green | 60505-7007-0 | 60505-7007-2 |
| Fentanyl Transdermal System-75 | 32.1 | 8.28 | Blue | 60505-7008-0 | 60505-7008-2 |
| Fentanyl Transdermal System-100 | 42.8 | 11.04 | Gray | 60505-7009-0 | 60505-7009-2 |
Store in original unopened blister. Store up to 25°C (77°F); excursions permitted to 15 to 30°C (59 to 86°F).
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide and Instructions for Use) Provide patients receiving fentanyl transdermal system patches the following information:
- Fentanyl transdermal system patches contain fentanyl, an opioid pain medicine that can cause serious breathing problems and death, especially if used in the wrong way and therefore should be taken only as directed. Instruct patients to call their doctor immediately or seek emergency medical help if they experience breathing problems while taking fentanyl transdermal system.
- Fentanyl transdermal system contains fentanyl which has a high potential for abuse. Instruct patients, family members, and caregivers to protect fentanyl transdermal system from theft or misuse in the work or home environment.
- Instruct patients to never give fentanyl transdermal system to anyone other than the individual for whom it was prescribed because of the risk of death or other serious medical problems to that person for whom it was not intended.
- Advise patients never to change the dose of fentanyl transdermal system or the number of patches applied to the skin unless instructed to do so by the prescribing healthcare professional.
- Warn patients of the potential for temperature-dependent increases in fentanyl release from the patch that could result in an overdose of fentanyl. Instruct patients to contact their healthcare provider if they develop a high fever. Instruct patients to:
- avoid strenuous exertion that can increase body temperature while wearing the patch
- avoid exposing the fentanyl transdermal system application site and surrounding area to direct external heat sources including heating pads, electric blankets, sunbathing, heat or tanning lamps, saunas, hot tubs or hot baths, and heated water beds.
- Keep fentanyl transdermal system in a secure place out of the reach of children due to the high risk of fatal respiratory depression. Fentanyl transdermal system can be accidentally transferred to children. Instruct patients to take special precautions to avoid accidental contact when holding or caring for children.
- If the patch dislodges and accidentally sticks to the skin of another person, to immediately take the patch off, wash the exposed area with water and seek medical attention for the accidentally exposed individual as accidental exposure may lead to death or other serious medical problems.
- To properly dispose of used and unneeded, unused fentanyl transdermal system, remove them from their blisters, fold them so that the adhesive side of the patch adheres to itself, and flush them down the toilet.
- Fentanyl transdermal system may impair mental and/or physical ability required for the performance of potentially hazardous tasks (e.g., driving, operating machinery). Instruct patients to refrain from any potentially dangerous activity when starting on fentanyl transdermal system or when their dose is being adjusted, until it is established that they have not been adversely affected.
- Advise women of childbearing potential who become, or are planning to become pregnant, to consult a healthcare provider prior to initiating or continuing therapy with fentanyl transdermal system.
- Instruct patients not to use alcohol or other CNS depressants (e.g. sleep medications, tranquilizers) while using fentanyl transdermal system because dangerous additive effects may occur, resulting in serious injury or death.
- Advise patients of the potential for severe constipation.
- When no longer needed, fentanyl transdermal system should not be stopped abruptly to avoid the risk of precipitating withdrawal symptoms.
- MRI: Skin burns have been reported at the patch site in several patients wearing an aluminized transdermal system during a magnetic resonance imaging scan (MRI). Because this fentanyl transdermal system contains aluminum, it is recommended to remove the patch before undergoing any MRI procedures.
Manufactured by: Manufactured for:
Aveva Drug Delivery Systems, Inc. Apotex Corp.
Miramar, FL 33025 Weston, FL 33326
3001944
Rev. July 2012
Medication Guide
Fentanyl Transdermal System Cll
Fentanyl transdermal system is:
- A strong prescription pain medicine that contains an opioid (narcotic) that is used to treat moderate to severe around-the-clock pain, in people who are already regularly using opioid pain medicine.
Important information about fentanyl transdermal system:
- Important information about fentanyl transdermal system: Get emergency help right away if you use too much fentanyl transdermal system (overdose). Fentanyl transdermal systemoverdose can cause life threatening breathing problems that can lead to death.
- Never give anyone else your fentanyl transdermal system. They could die from using it. Store fentanyl transdermal system away from children and in a safe place to prevent stealing or abuse. Selling or giving away fentanyl transdermal system is against the law.
Do not use fentanyl transdermal system if you have:
- severe asthma, trouble breathing, or other lung problems.
- a bowel blockage or have narrowing of the stomach or intestines.
Before applying fentanyl transdermal system, tell your healthcare provider if you have a history of:
- head injury, seizures
- liver, kidney, thyroid problems
- problems urinating
- pancreas or gallbladder problems
- abuse of street or prescription drugs, alcohol addiction, or mental health problems.
Tell your healthcare provider if you:
- have a fever
- are pregnant or planning to become pregnant. Fentanyl transdermal system may harm your unborn baby.
- are breastfeeding. Fentanyl passes into breast milk and may harm your baby.
- are taking prescription or over-the-counter medicines, vitamins, or herbal supplements.
When using fentanyl transdermal system:
- Do not change your dose. Apply fentanyl transdermal system exactly as prescribed by your healthcare provider.
- See the detailed Instructions for Use for information about how to apply and dispose of the fentanyl transdermal system patch.
- Do not wear more than 1 patch at the same time unless your healthcare provider tells you to.
- Call your healthcare provider if the dose you are using does not control your pain.
- Do not stop using fentanyl transdermal system without talking to your healthcare provider.
While using fentanyl transdermal system Do Not:
- · Take hot baths or sunbathe, use hot tubs, saunas, heating pads, electric blankets, heated waterbeds, or tanning lamps, or engage in exercise that increases your body temperature. These can cause an overdose that can lead to death. ·
- Drive or operate heavy machinery, until you know how fentanyl transdermal system affects you. Fentanyl transdermal system can make you sleepy, dizzy, or lightheaded.
- Drink alcohol or use prescription or over-the-counter medicines that contain alcohol. Wear this fentanyl transdermal system during any magnetic resonance imaging (MRI) procedures to avoid possible burns.
The possible side effects of fentanyl transdermal system are:
- constipation, nausea, sleepiness, vomiting, tiredness, headache, dizziness, abdominal pain, itching, redness, or rash where the patch is applied. Call your healthcare provider if you have any of these symptoms and they are severe.
Get emergency medical help if you have:
- trouble breathing, shortness of breath, fast heartbeat, chest pain, swelling of your face, tongue or throat, extreme drowsiness, or you are feeling faint.
These are not all the possible side effects of fentanyl transdermal system. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. For more information go to dailymed.nlm.nih.gov
Manufactured by:
Aveva Drug Delivery Systems, Inc., Miramar, FL 33025;
Manufactured for:
Apotex Corp., Weston, FL 33326, drugsafety@apotex.com or call
1-888-333-6127, 9 A.M. to 5 P.M., Eastern Time, Monday through Friday.
This Medication Guide has been approved by the U.S. FDA. Issue: July 2012
Instructions for Applying a Fentanyl Transdermal System Patch
Rx only

Before Applying Fentanyl Transdermal System
- Each fentanyl transdermal system patch is sealed in its own protective blister. Do not remove a fentanyl transdermal system patch from the blister until you are ready to use it.
- Do not use a fentanyl transdermal system patch if the seal is broken or the patch is cut, damaged or changed in any way.
- Fentanyl transdermal system patches are available in 4 different doses and patch sizes. Make sure you have the right dose patch or patches that have been prescribed for you.
Applying a Fentanyl Transdermal System Patch
-
Skin Areas Where the Fentanyl Transdermal System Patch May Be Applied:
For adults- · Put the patch on the chest, back, flank (sides of the waist), or upper arm in a place where there is no hair (see Figures 1 to 4).
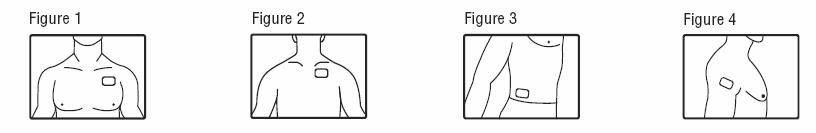
- Put the patch on the upper back (see Figure 2). This will lower the chances that the child will remove the patch and put it in their mouth.
- · Do not put a fentanyl transdermal system patch on skin that is very oily, burned, broken out, cut, irritated, or damaged in any way.
- Avoid sensitive areas or those that move around a lot. If there is hair, do not shave (shaving irritates the skin). Instead, clip hair as close to the skin as possible (see Figure 5).
- Talk to your doctor if you have questions about skin application sites.
- · Put the patch on the chest, back, flank (sides of the waist), or upper arm in a place where there is no hair (see Figures 1 to 4).
-
Prepare to Apply a Fentanyl Transdermal System Patch:
- Choose the time of day that is best for you to apply the fentanyl transdermal system. Change it at about the same time of day (3 days or 72 hours after you apply the patch) or as directed by your doctor.
- Do not wear more than one fentanyl transdermal system patch at a time unless your doctor tells you to do so. Before putting on a new fentanyl transdermal system patch, remove the patch you have been wearing.
- Clean the skin area with clear water only. Pat skin completely dry. Do not use anything on the skin such as soaps, lotions, oils, or alcohol before the patch is applied.
-
Open the blister: Hold the blister package so that the fentanyl transdermal system patch is visible, then cut through the blister package taking care not to cut through the fentanyl transdermal system, and remove the fentanyl transdermal system patch. Each fentanyl transdermal system patch is sealed in its own protective blister. Do not remove the fentanyl transdermal system patch from the blister until you are ready to use it (See Figure 6).
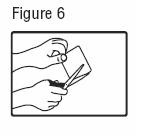
-
Peel: Peel off both parts of the protective liner from the patch. Each fentanyl transdermal system patch has a clear plastic backing that can be peeled off in two pieces. This covers the sticky side of the patch. Carefully peel this backing off. Throw the clear plastic backing away. Touch the sticky side of a fentanyl transdermal system patch as little as possible (see Figure 7).
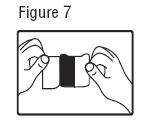
-
Press: Press the patch onto the chosen skin site with the palm of your hand and hold there for at least 30 seconds (see Figure 8). Make sure it sticks well, especially at the edges.

- Fentanyl transdermal system may not stick to all patients. You need to check the patches often to make sure that they are sticking well to the skin.
- If the patch falls off right away after applying, throw it away and put a new one on at a different skin site (see Disposing a Fentanyl Transdermal System Patch).
- If you have a problem with the patch not sticking
- Apply first aid tape only to the edges of the patch.
- If you continue to have problems with the patch sticking, you may cover the patch with Bioclusive™. This is a special see-through adhesive dressing. Never cover a fentanyl transdermal system with any other bandage or tape. Remove the backing from the Bioclusive™ dressing and place it carefully over the fentanyl transdermal system patch, smoothing it over the patch and your skin
- If your patch falls off later, but before 3 days (72 hours) of use, discard it properly (see Disposing a Fentanyl Transdermal System Patch) and put a new one on at a different skin site. Be sure to let your doctor know that this has happened, and do not replace the new patch until 3 days (72 hours) after you put it on (or as directed by your doctor).
- Wash your hands when you have finished applying a fentanyl transdermal system patch.
- Remove a fentanyl transdermal system patch after wearing it for 3 days (72 hours) (see Disposing a Fentanyl Transdermal System Patch). Choose a different place on the skin to apply a new fentanyl transdermal system patch and repeat Steps 2 through 6.
Do not apply the new patch to the same place as the last one.
Water and Fentanyl Transdermal System- You can bathe, swim or shower while you are wearing a fentanyl transdermal system patch. If the patch falls off before 3 days (72 hours) after application, discard it properly (see Disposing a Fentanyl Transdermal System Patch) and put a new one on at a different skin site. Be sure to let your doctor know that this has happened, and do not replace the new patch until 3 days (72 hours) after you put it on (or as directed by your doctor).
Disposing a Fentanyl Transdermal System Patch
- Fold the used fentanyl transdermal system patch in half so that the sticky side sticks to itself (Figure 9). Flush the used fentanyl transdermal system down the toilet right away (Figure 10). A used fentanyl transdermal system patch CAN be VERY dangerous for or even lead to death in babies, children, pets, and adults who have not been prescribed fentanyl transdermal system.
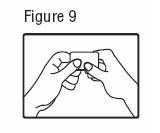
- Throw away any fentanyl transdermal system patches that are left over from your prescription as soon as they are no longer needed. Remove the leftover patches from their protective blister and remove the protective liner. Fold the patches in half with the sticky sides together, and flush the patches down the toilet. Do not flush the blister or the protective liner down the toilet. These items can be thrown away in a trashcan.
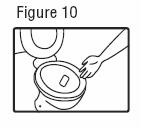
Bioclusive™ is a trademark of Ethicon, Inc.
Manufactured By: Manufactured For:
Aveva Drug Delivery Systems, Inc. Apotex Corp.
Miramar, FL 33025 Weston, FL 33326
3001944
Rev. 07/2012
PRINCIPAL DISPLAY PANEL - 25 mcg/hr Carton

Front Panel
NDC 60505-7006-2
Five (25 mcg/hr) Systems
FENTANYL TRANSDERMAL SYSTEM CII
25 mcg/hr
Rx Only
To avoid possible burns, remove the Fentanyl Transdermal Systembefore undergoing any MRI (magnetic resonance imaging) procedure.
In vivo delivery of 25 mcg/hr fentanyl for 72 hours
Because it can cause trouble breathing which can be fatal,
DO NOT USE FENTANYL TRANSDERMAL SYSTEM:
- For short term or any post-operative pain, or occasional pain
- For mild pain or pain that can be treated with non-opioid or as-needed opioid medication
- Unless you have been using other narcotic opioid medicines (must be opioid tolerant)
Each transdermal system contains: 2.76 mg fentanyl
DO NOT USE IF SEAL ON BLISTER IS BROKEN
KEEP OUT OF REACH OF CHILDREN
Read enclosed FENTANYL TRANSDERMAL SYSTEM Medication Guide for important safety information.
ONLY for pain requiring opioid medicine around-the-clock
Apotex Corp.
Back Panel
Inactive Ingredients: isopropyl myristate, octyldodecanol, polybutene, and polyisobutene adhesive.
Usual Dosage: For information for use, see accompanying product literature.
Apply immediately upon removal from blister and after removal of the protective liner. Do notexpose area to heat. Store in original unopened blister. Store up to 25ºC (77ºF); excursionspermitted to 15-30ºC (59-86ºF).
See Medication Guide for important safety information.
For your convenience in recording narcotic use,
INITIAL/DATE
1._______________ 2._______________ 3._______________
4._______________ 5._______________
For questions regarding this product, please call Apotex Corp. at 1-888-333-6127, 9 A.M. to 5 P.M.Eastern Time, Monday through Friday. If this is a medical emergency, please call 911..
Manufactured by:
Aveva Drug Delivery Systems
An Apotex Company.
Miramar, FL 33
Manufactured for:
Apotex Corp.
Weston, FL 33326
3001940 Rev.06/2012
PRINCIPAL DISPLAY PANEL - 25 mcg/hr Pouch

Front Panel
NDC 60505-7006-0
One (25 mcg/hr) Systems
FENTANYL TRANSDERMAL SYSTEM 25mcg/h CII
Rx only
In vivo delivery of 25 mcg/hr fentanyl for 72 hours
To avoid possible burns, remove the Fentanyl Transdermal System beforeundergoing any MRI (magnetic resonance imaging) procedure.
Because it can cause trouble breathing which can be fatal,
DO NOT USE FENTANYL TRANSDERMAL SYSTEM:
- For short term or any post-operative pain, or occasional pain
- For mild pain or pain that can be treated with non-opioid or as-needed opioid medication
- Unless you have been using other narcotic opioid medicines (must be opioid tolerant)
Each transdermal system contains: 2.76 mg fentanyl
KEEP OUT OF REACH OF CHILDREN
Read enclosed FENTANYL TRANSDERMAL SYSTEM
Medication Guide for important safety information.
Inactive Ingredients: isopropyl myristate, octyldodecanol, polybutene, andpolyisobutene adhesive.
Dosage:For information for use, see accompanying product literature.
Apply immediately upon removal from blister and after removal of the protective liner.
Do not expose area to heat. Store in original unopened blister.
Store up to 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
DO NOT USE IF SEAL ON POUCH IS BROKEN.
KEEP OUT OF REACH OF CHILDREN.
Read enclosed Fentanyl Transdermal System
Medication Guide for important safety information.
Manufactured for: Apotex Corp. Weston, FL 33326
ONLY for pain requiring opioid medicine around-the-clock
Apotex Corp
Rev. 07/2012
4001380.
PRINCIPAL DISPLAY PANEL - 50 mcg/hr Carton

Front Panel
NDC 60505-7007-2
Five (50 mcg/hr) Systems
FENTANYL TRANSDERMAL SYSTEM CII
50 mcg/hr
Rx Only
To avoid possible burns, remove the Fentanyl Transdermal Systembefore undergoing any MRI (magnetic resonance imaging) procedure.
In vivo delivery of 50 mcg/hr fentanyl for 72 hours
Because it can cause trouble breathing which can be fatal,
DO NOT USE FENTANYL TRANSDERMAL SYSTEM:
- For short term or any post-operative pain, or occasional pain
- For mild pain or pain that can be treated with non-opioid or as-needed opioid medication
- Unless you have been using other narcotic opioid medicines (must be opioid tolerant)
Each transdermal system contains:2.76 mg fentanyl
DO NOT USE IF SEAL ON BLISTER IS BROKEN
KEEP OUT OF REACH OF CHILDREN
Read enclosed FENTANYL TRANSDERMAL SYSTEM Medication Guide for important safety information.
ONLY for pain requiring opioid medicine around-the-clock
Apotex Corp.
Back Panel
Inactive Ingredients: isopropyl myristate, octyldodecanol, polybutene, and polyisobutene adhesive.
Usual Dosage: For information for use, see accompanying product literature.
Apply immediately upon removal from blister and after removal of the protective liner. Do notexpose area to heat. Store in original unopened blister. Store up to 25ºC (77ºF); excursionspermitted to 15-30ºC (59-86ºF).
See Medication Guide for important safety information.
For your convenience in recording narcotic use,
INITIAL/DATE
1._______________ 2._______________ 3._______________
4._______________ 5._______________
For questions regarding this product, please call Apotex Corp. at 1-888-333-6127, 9 A.M. to 5 P.M.Eastern Time, Monday through Friday. If this is a medical emergency, please call 911..
Manufactured by:
Aveva Drug Delivery Systems
An Apotex Company.
Miramar, FL 33
Manufactured for:
Apotex Corp.
Weston, FL 33326
ONLY for pain requiring opioid medicine around-the-clock
Apotex Corp.
3001940 Rev.06/2012
PRINCIPAL DISPLAY PANEL - 50 mcg/hr Pouch

Front Panel
NDC 60505-7007-0
One (50 mcg/hr) Systems
FENTANYL TRANSDERMAL SYSTEM CII
50 mcg/h
Rx only
In vivo delivery of 50 mcg/hr fentanyl for 72 hours
To avoid possible burns, remove the Fentanyl Transdermal System beforeundergoing any MRI (magnetic resonance imaging) procedure.
Because it can cause trouble breathing which can be fatal,
DO NOT USE FENTANYL TRANSDERMAL SYSTEM:
- For short term or any post-operative pain, or occasional pain
- For mild pain or pain that can be treated with non-opioid or as-needed opioid medication
- Unless you have been using other narcotic opioid medicines (must be opioid tolerant)
Each transdermal system contains: 2.76 mg fentanyl
KEEP OUT OF REACH OF CHILDREN
Read enclosed FENTANYL TRANSDERMAL SYSTEM
Medication Guide for important safety information.
Inactive Ingredients: isopropyl myristate, octyldodecanol, polybutene, andpolyisobutene adhesive.
Dosage:For information for use, see accompanying product literature.
Apply immediately upon removal from blister and after removal of the protective liner.
Do not expose area to heat. Store in original unopened blister.
Store up to 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
DO NOT USE IF SEAL ON POUCH IS BROKEN.
KEEP OUT OF REACH OF CHILDREN.
Read enclosed Fentanyl Transdermal System
Medication Guide for important safety information.
Manufactured for: Apotex Corp. Weston, FL 33326
ONLY for pain requiring opioid medicine around-the-clock
Apotex Corp.
Rev. 07/2012
4001380
PRINCIPAL DISPLAY PANEL - 75 mcg/hr Carton

Front Panel
NDC 60505-7008-2
Five (75 mcg/hr) Systems
FENTANYL TRANSDERMAL SYSTEM CII
75 mcg/hr
Rx Only
In vivo delivery of 25 mcg/hr fentanyl for 72 hours
Because it can cause trouble breathing which can be fatal,
DO NOT USE FENTANYL TRANSDERMAL SYSTEM:
- For short term or any post-operative pain, or occasional pain
- For mild pain or pain that can be treated with non-opioid or as-needed opioid medication
- Unless you have been using other narcotic opioid medicines (must be opioid tolerant)
Each transdermal system contains: 2.76 mg fentanyl
DO NOT USE IF SEAL ON BLISTER IS BROKEN
KEEP OUT OF REACH OF CHILDREN
Read enclosed FENTANYL TRANSDERMAL SYSTEM
Medication Guide for important safety information.
ONLY for pain requiring opioid medicine around-the-clock
Apotex Corp.
Back Panel
Inactive Ingredients: isopropyl myristate, octyldodecanol, polybutene, and polyisobutene adhesive.
Usual Dosage: For information for use, see accompanying product literature.
Apply immediately upon removal from blister and after removal of the protective liner. Do notexpose area to heat. Store in original unopened blister. Store up to 25ºC (77ºF); excursionspermitted to 15-30ºC (59-86ºF).
See Medication Guide for important safety information.
For your convenience in recording narcotic use,
INITIAL/DATE
1._______________ 2._______________ 3._______________
4._______________ 5._______________
For questions regarding this product, please call Apotex Corp. at 1-888-333-6127, 9 A.M. to 5 P.M.Eastern Time, Monday through Friday. If this is a medical emergency, please call 911..
Manufactured by:
Aveva Drug Delivery Systems
An Apotex Company.
Miramar, FL 33
Manufactured for:
Apotex Corp.
Weston, FL 33326
ONLY for pain requiring opioid medicine around-the-clock
Apotex Corp.
3001940 Rev.06/2012
PRINCIPAL DISPLAY PANEL - 75 mcg/hr Pouch

Front Panel
NDC 60505-7008-0
One (75 mcg/hr) Systems
FENTANYL TRANSDERMAL SYSTEM CII
75 mcg/h
Rx only
In vivo delivery of 75 mcg/hr fentanyl for 72 hours
To avoid possible burns, remove the Fentanyl Transdermal System beforeundergoing any MRI (magnetic resonance imaging) procedure.
Because it can cause trouble breathing which can be fatal,
DO NOT USE FENTANYL TRANSDERMAL SYSTEM:
- For short term or any post-operative pain, or occasional pain
- For mild pain or pain that can be treated with non-opioid or as-needed opioid medication
- Unless you have been using other narcotic opioid medicines (must be opioid tolerant)
Each transdermal system contains: 2.76 mg fentanyl
KEEP OUT OF REACH OF CHILDREN
Read enclosed FENTANYL TRANSDERMAL SYSTEM
Medication Guide for important safety information.
Inactive Ingredients: isopropyl myristate, octyldodecanol, polybutene, andpolyisobutene adhesive.
Dosage:For information for use, see accompanying product literature.
Apply immediately upon removal from blister and after removal of the protective liner.
Do not expose area to heat. Store in original unopened blister.
Store up to 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
DO NOT USE IF SEAL ON POUCH IS BROKEN.
KEEP OUT OF REACH OF CHILDREN.
Read enclosed Fentanyl Transdermal System
Medication Guide for important safety information.
Manufactured for: Apotex Corp. Weston, FL 33326
Rev. 07/2012
4001380
PRINCIPAL DISPLAY PANEL - 100 mcg/hr Carton

Front Panel
NDC 60505-7009-2
Five (100 mcg/hr) Systems
FENTANYL TRANSDERMAL SYSTEM CII
100 mcg/hr
Rx Only
In vivo delivery of 100 mcg/hr fentanyl for 72 hours
Because it can cause trouble breathing which can be fatal,
DO NOT USE FENTANYL TRANSDERMAL SYSTEM:
- For short term or any post-operative pain, or occasional pain
- For mild pain or pain that can be treated with non-opioid or as-needed opioid medication
- Unless you have been using other narcotic opioid medicines (must be opioid tolerant)
Each transdermal system contains: 2.76 mg fentanyl
DO NOT USE IF SEAL ON BLISTER IS BROKEN
KEEP OUT OF REACH OF CHILDREN
Read enclosed FENTANYL TRANSDERMAL SYSTEM
Medication Guide for important safety information.
ONLY for pain requiring opioid medicine around-the-clock
Apotex Corp.
Back Panel
Inactive Ingredients: isopropyl myristate, octyldodecanol, polybutene, and polyisobutene adhesive.
Usual Dosage: For information for use, see accompanying product literature.
Apply immediately upon removal from blister and after removal of the protective liner. Do notexpose area to heat. Store in original unopened blister. Store up to 25ºC (77ºF); excursionspermitted to 15-30ºC (59-86ºF).
See Medication Guide for important safety information.
For your convenience in recording narcotic use,
INITIAL/DATE
1._______________ 2._______________ 3._______________
4._______________ 5._______________
For questions regarding this product, please call Apotex Corp. at 1-888-333-6127, 9 A.M. to 5 P.M.Eastern Time, Monday through Friday. If this is a medical emergency, please call 911..
Manufactured by:
Aveva Drug Delivery Systems
An Apotex Company.
Miramar, FL 33
Manufactured for:
Apotex Corp.
Weston, FL 33326
ONLY for pain requiring opioid medicine around-the-clock
Apotex Corp.
3001940 Rev.06/2012
PRINCIPAL DISPLAY PANEL - 100 mcg/hr Pouch

Front Panel
NDC 60505-7009-0
One (100 mcg/hr) Systems
FENTANYL TRANSDERMAL SYSTEM 100 mcg/h CII
Rx only
In vivo delivery of 100 mcg/hr fentanyl for 72 hours
To avoid possible burns, remove the Fentanyl Transdermal System beforeundergoing any MRI (magnetic resonance imaging) procedure.
Because it can cause trouble breathing which can be fatal,
DO NOT USE FENTANYL TRANSDERMAL SYSTEM:
- For short term or any post-operative pain, or occasional pain
- For mild pain or pain that can be treated with non-opioid or as-needed opioid medication
- Unless you have been using other narcotic opioid medicines (must be opioid tolerant)
Each transdermal system contains: 2.76 mg fentanyl
KEEP OUT OF REACH OF CHILDREN
Read enclosed FENTANYL TRANSDERMAL SYSTEM
Medication Guide for important safety information.
Inactive Ingredients: isopropyl myristate, octyldodecanol, polybutene, andpolyisobutene adhesive.
Dosage:For information for use, see accompanying product literature.
Apply immediately upon removal from blister and after removal of the protective liner.
Do not expose area to heat. Store in original unopened blister.
Store up to 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
DO NOT USE IF SEAL ON POUCH IS BROKEN.
KEEP OUT OF REACH OF CHILDREN.
Read enclosed Fentanyl Transdermal System
Medication Guide for important safety information.
Manufactured for: Apotex Corp. Weston, FL 33326
Rev. 07/2012
4001380
FENTANYLFENTANYL PATCH, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
FENTANYLFENTANYL PATCH, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
FENTANYLFENTANYL PATCH, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
FENTANYLFENTANYL PATCH, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||