FELODIPINE
Felodipine Extended-Release Tablets, USP Rx only
FULL PRESCRIBING INFORMATION: CONTENTS*
- FELODIPINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- Clinical Studies
- FELODIPINE INDICATIONS AND USAGE
- FELODIPINE CONTRAINDICATIONS
- PRECAUTIONS
- FELODIPINE ADVERSE REACTIONS
- OVERDOSAGE
- FELODIPINE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
FELODIPINE DESCRIPTION
181924
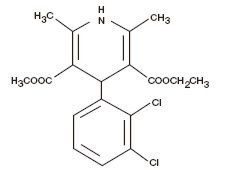
CLINICAL PHARMACOLOGY
Mechanism of Action
++In vitroin vitro
Pharmacokinetics and Metabolism
1/2
1/250
50
14
max
Geriatric Use
Hepatic Dysfunction
Cardiovascular Effects
Renal/Endocrine Effects
Clinical Studies
| Dose | N | Systolic/Diastolic Mean Peak Response | Mean Trough Response | Trough/Peak Ratios (%s) |
|---|---|---|---|---|
| Study 1 (8 weeks) | ||||
| 2.5 mg | 68 | 9.4/4.7 | 2.7/2.5 | 29/53 |
| 5 mg | 69 | 9.5/6.3 | 2.4/3.7 | 25/59 |
| 10 mg | 67 | 18.0/10.8 | 10.0/6.0 | 56/56 |
| Study 2 (4 weeks) | ||||
| 10 mg | 50 | 5.3/7.2 | 1.5/3.2 | 33/40** |
| 20 mg | 50 | 11.3/10.2 | 4.5/3.2 | 43/34** |
FELODIPINE INDICATIONS AND USAGE
FELODIPINE CONTRAINDICATIONS
PRECAUTIONS
General
Hypotension-Heart Failure
Patients with Impaired Liver Function
Peripheral Edema-
Information for Patients
Drug Interactions
CYP3A4 InhibitorsItraconazolemax
Erythromycinmax
Grapefruit juicemax
Cimetidinemax
Beta-Blocking Agentsmax
Digoxin
Anticonvulsants
Tacrolimus
Other Concomitant Therapy
Interaction with Food
Carcinogenesis, Mutagenesis, Impairment of Fertility
**2**2**2
in vitroin vivo**2in vitro
**2
Pregnancy
Teratogenic Effects**2
Nonteratogenic Effects**2
2
Nursing Mothers
Pediatric Use
Geriatric Use:
FELODIPINE ADVERSE REACTIONS
| Body System Adverse Events |
Placebo N=334 |
2.5 mg N=255 |
5 mg N=581 |
10 mg N=408 |
|
|---|---|---|---|---|---|
| Body as a Whole | |||||
| Peripheral Edema | 3.3 (0.0) | 2.0 (0.0) | 8.8 (2.2) | 17.4 (2.5) | |
| Asthenia | 3.3 (0.0) | 3.9 (0.0) | 3.3 (0.0) | 2.2 (0.0) | |
| Warm Sensation | 0.0 (0.0) | 0.0 (0.0) | 0.9 (0.2) | 1.5 (0.0) | |
| Cardiovascular | |||||
| Palpitation | 2.4 (0.0) | 0.4 (0.0) | 1.4 (0.3) | 2.5 (0.5) | |
| Digestive | |||||
| Nausea | 1.5 (0.9) | 1.2 (0.0) | 1.7 (0.3) | 1.0 (0.7) | |
| Dyspepsia | 1.2 (0.0) | 3.9 (0.0) | 0.7 (0.0) | 0.5 (0.0) | |
| Constipation | 0.9 (0.0) | 1.2 (0.0) | 0.3 (0.0) | 1.5 (0.2) | |
| Nervous | |||||
| Headache | 10.2 (0.9) | 10.6 (0.4) | 11.0 (1.7) | 14.7 (2.0) | |
| Dizziness | 2.7 (0.3) | 2.7 (0.0) | 3.6 (0.5) | 3.7 (0.5) | |
| Paresthesia | 1.5 (0.3) | 1.6 (0.0) | 1.2 (0.0) | 1.2 (0.2) | |
| Respiratory | |||||
| Upper Respiratory Infection | 1.8 (0.0) | 3.9 (0.0) | 1.9 (0.0) | 0.7 (0.0) | |
| Cough | 0.3 (0.0) | 0.8 (0.0) | 1.2 (0.0) | 1.7 (0.0) | |
| Rhinorrhea | 0.0 (0.0) | 1.6 (0.0) | 0.2 (0.0) | 0.2 (0.0) | |
| Sneezing | 0.0 (0.0) | 1.6 (0.0) | 0.0 (0.0) | 0.0 (0.0) | |
| Skin | |||||
| Rash | 0.9 (0.0) | 2.0 (0.0) | 0.2 (0.0) | 0.2 (0.0) | |
| Flushing | 0.9 (0.3) | 3.9 (0.0) | 5.3 (0.7) | 6.9 (1.2) |
Body as a Whole: Cardiovascular: Myocardial infarction, hypotensionsyncope, angina pectoris, arrhythmia Digestive: Endocrine: Hematologic: Anemia; Metabolic: Musculoskeletal: Nervous/Psychiatric: Respiratory: Skin: Angioedemaleukocytoclastic vasculitis Special Senses: Urogenital:
Gingival Hyperplasia
Clinical Laboratory Test Findings
Serum ElectrolytesSerum Glucose
Liver Enzymes
OVERDOSAGE
Physicians' Desk Reference (PDR)
FELODIPINE DOSAGE AND ADMINISTRATION
Geriatric Use
Patients with Impaired Liver Function
HOW SUPPLIED
Storage:
Manufactured by:
Distributed by:

FELODIPINEFELODIPINE TABLET, FILM COATED, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
FELODIPINEFELODIPINE TABLET, FILM COATED, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
FELODIPINEFELODIPINE TABLET, FILM COATED, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!