Famotidine
NorthStar RxLLC
Alembic Pharmaceuticals Limited
Famotidine Tablets, USP Rx only
FULL PRESCRIBING INFORMATION: CONTENTS*
- FAMOTIDINE DESCRIPTION
- CLINICAL PHARMACOLOGY IN ADULTS
- CLINICAL PHARMACOLOGY IN PEDIATRIC PATIENTS
- FAMOTIDINE INDICATIONS AND USAGE
- FAMOTIDINE CONTRAINDICATIONS
- PRECAUTIONS
- FAMOTIDINE ADVERSE REACTIONS
- OVERDOSAGE
- FAMOTIDINE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
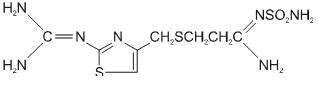
FAMOTIDINE DESCRIPTION
N815723
CLINICAL PHARMACOLOGY IN ADULTS
ADVERSE REACTIONS
PRECAUTIONSDOSAGE AND ADMINISTRATION
PRECAUTIONS
Clinical Studies
Duodenal Ulcer
|
Table 1 |
|||
|
Outpatients With Endoscopically |
|||
|
Confirmed Healed Duodenal Ulcers |
|||
|
|
Famotidine |
Famotidine |
Placebo |
|
|
40 mg h.s. |
20 mg b.i.d. |
h.s. |
|
|
(N = 89) |
(N = 84) |
(N = 97) |
|
|
|
|
|
|
Week 2 |
**32% |
**38% |
17% |
|
Week 4 |
**70% |
**67% |
31% |
Long-Term Maintenance
|
Table 2 |
||||
|
Patients with Endoscopically Confirmed |
||||
|
Healed Gastric Ulcers |
||||
|
|
U.S.Study |
International Study |
||
|
|
Famotidine |
Placebo |
Famotidine |
Placebo |
|
|
(N = 74) 40 mg h.s. |
(N = 75) h.s. |
(N = 149) 40 mg h.s. |
(N = 145) h.s. |
|
Week 4 |
45% |
39% |
†47% |
31% |
|
Week 6 |
†66% |
44% |
†65% |
46% |
|
Week 8 |
***78% |
64% |
†80% |
54% |
|
Table 3 |
|||
|
% Successful Symptomatic Outcome |
|||
|
|
Famotidine |
Famotidine |
|
|
|
20 mg b.i.d |
40 mg h.s. |
Placebo |
|
|
(N = 154) |
(N = 149) |
(N = 73) |
|
|
|
|
|
|
Week 6 |
82†† |
69 |
62 |
|
Table 4 |
|||
|
% Endoscopic Healing - U.S. Study |
|||
|
|
Famotidine |
Famotidine |
|
|
|
40 mg b.i.d |
20 mg b.i.d |
Placebo |
|
|
(N = 127) |
(N = 125) |
(N = 66) |
|
|
|
|
|
|
Week 6 |
48†††,‡‡ |
32 |
18 |
|
Week 12 |
69†††,‡ |
54††† |
29 |
In the international study, when famotidine 40 mg p.o. b.i.d., was compared to ranitidine 150 mg p.o. b.i.d., a statistically significantly greater percentage of healing was observed with famotidine 40 mg b.i.d. at week 12 (Table 5). There was, however, no significant difference among treatments in symptom relief.
|
Table 5 |
|||
|
% Endoscopic Healing - International Study. |
|||
|
|
Famotidine |
Famotidine |
Ranitidine |
|
|
40 mg b.i.d |
20 mg b.i.d. |
150 mg b.i.d. |
|
|
(N = 175) |
(N = 93) |
(N = 172) |
|
|
|
|
|
|
Week 6 |
48 |
52 |
42 |
|
Week 12 |
71‡‡‡ |
68 |
60 |
CLINICAL PHARMACOLOGY IN PEDIATRIC PATIENTS
|
Table 6 |
||||
|
Pharmacokinetic Parametersa of Intravenous Famotidine |
||||
|
Age (N= number of patients) |
Area Under the Curve (AUC) (ng-hr/ml) |
Total Clearance (CI) (L/hr/kg) |
Volume of Distribution(Vd) (L/kg) |
Elimination Half-life (T1/2) (hours) |
|
0-1monthsc |
NA |
0.13±0.06 |
1.4±0.4 |
10.5±5.4 |
|
(N=10) |
|
|
|
|
|
0-3 monthsd |
2688±847 |
0.21±0.06 |
1.8±0.3 |
8.1±3.5 |
|
(N=6) |
|
|
|
|
|
>3-12 monthsd |
1160±474 |
0.49±0.17 |
2.3±0.7 |
4.5±1.1 |
|
(N=11) |
|
|
|
|
|
1-11 yrs |
1089±834 |
0.54±0.34 |
2.07±1.49 |
3.38±2.60 |
|
(N=20) |
|
|
|
|
|
11-15 yrs |
1140±320 |
0.48±0.14 |
1.5±0.4 |
2.3±0.4 |
|
(N=6) |
|
|
|
|
|
Adult |
1726b |
0.39±0.14 |
1.3±0.2 |
2.83±0.99 |
|
(N=16) |
|
|
|
|
a
b
c
d
max
|
Table 7 |
|
|
Pharmacodynamics of famotidine using the sigmoid Emax model |
|
|
Pediatric Patients |
EC50 (ng/mL)* 26 ± 13 |
|
Data from one study |
|
|
a) healthy adult subjects |
26.5 ± 10.3 |
|
b) adult patients with upper GI bleeding |
18.7 ± 10.8 |
|
Table 8 |
|||
|
Dosage |
Route |
Effecta |
Number of Patients (age range) |
|
0.5 mg/kg, single dose |
I.V. |
gastric pH >4 for 19.5 hours(17.3, 21.8) c |
11 (5 to 19 days) |
|
0.3 mg/kg, single dose |
I.V. |
gastric pH >3.5 for 8.7 ± 4.7b hours |
6 (2 to 7 years) |
|
0.4 to 0.8 mg/kg |
I.V. |
gastric pH > 4 for 6 to 9 hours |
18 (2 to 69 months) |
|
0.5 mg/kg, single dose |
I.V. |
a >2 pH unit increase above baseline in gastric pH for >8 hours |
9 (2 to 13 years) |
|
0.5 mg/kg b.i.d. |
I.V. |
gastric pH >5 for 13.5 ± 1.8b hours |
4 (6 to 15 years) |
|
0.5 mg/kg b.i.d. |
oral |
gastric pH >5 for 5.0 ± 1.1b hours |
4 (11 to 15 years) |
a
b
c
FAMOTIDINE INDICATIONS AND USAGE
Short term treatment of active duodenal ulcer.
Maintenance therapy for duodenal ulcer patients at reduced dosage after healing of an active ulcer.
Short term treatment of active benign gastric ulcer.
Short term treatment of gastroesophageal reflux disease (GERD). CLINICAL PHARMACOLOGY IN ADULTS
CLINICAL PHARMACOLOGY IN ADULTS
Treatment of pathological hypersecretory conditions (e.g., Zollinger-Ellison Syndrome, multiple endocrine adenomas) CLINICAL PHARMACOLOGY IN ADULTS.
FAMOTIDINE CONTRAINDICATIONS
Hypersensitivity to any component of these products. Cross sensitivity in this class of compounds has been observed. Therefore, famotidine should not be administered to patients with a history of hypersensitivity to other H2-receptor antagonists.
PRECAUTIONS
General
Patients with Moderate or Severe Renal Insufficiency
CLINICAL PHARMACOLOGY IN ADULTSDOSAGE AND ADMINISTRATION
Drug Interactions
No drug interactions have been identified. Studies with famotidine in man, in animal models, and in vitro have shown no significant interference with the disposition of compounds metabolized by the hepatic microsomal enzymes, e.g., cytochrome P450 system. Compounds tested in man include warfarin, theophylline, phenytoin, diazepam, aminopyrine and antipyrine. Indocyanine green as an index of hepatic drug extraction has been tested and no significant effects have been found.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Salmonella typhimurium Escherichia coli in vivo
Pregnancy
Nursing Mothers
Pediatric Patients <1 year of age
CLINICAL PHARMACOLOGY IN PEDIATRIC PATIENTS
ADVERSE REACTIONS
Pediatric Patients 1 - 16 years of age
Geriatric Use
CLINICAL PHARMACOLOGY IN ADULTSPRECAUTIONSPatients with Moderate or Severe Renal Insufficiency DOSAGE AND ADMINISTRATIONDosage Adjustment forPatients with Moderate or Severe Renal Insufficiency
FAMOTIDINE ADVERSE REACTIONS
Pediatric Patients
OVERDOSAGE
ADVERSE REACTIONS
FAMOTIDINE DOSAGE AND ADMINISTRATION
DuodenalUlcer
Acute Therapy:
Maintenance Therapy:
Benign Gastric Ulcer
Acute Therapy:
Gastroesophageal Reflux Disease (GERD)
CLINICAL PHARMACOLOGY IN ADULTSClinical Studies
Dosage for Pediatric Patients <1 year of age
Gastroesophageal Reflux Disease (GERD)
PRECAUTIONS
Dosage for Pediatric Patients 1-16 years of age
PRECAUTIONS
Pediatric Patients 1-16 years of age
Peptic ulcer
Gastroesophageal Reflux Disease with or without esophagitis including erosions and ulcerations
Pathological Hypersecretory Conditions (e.g., Zollinger-Ellison Syndrome, Multiple Endocrine Adenomas)
Concomitant Use of Antacids
Dosage Adjustment for Patients with Moderate or Severe Renal Insufficiency
HOW SUPPLIED
Storage
PRINCIPAL DISPLAY PANEL
NDC 16714-361-01
Each Film Coated Tablet Contains:
Famotidine USP 20 mg

PRINCIPAL DISPLAY PANEL
Famotidine Tablets USP 40 mg (30 Tablets in 1 Bottle)
NDC 16714-362-01
Each Film Coated Tablet Contains:
Famotidine USP 40 mg

FamotidineFamotidine TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
FamotidineFamotidine TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||