Famotidine
Rx only
FULL PRESCRIBING INFORMATION: CONTENTS*
- FAMOTIDINE DESCRIPTION
- CLINICAL PHARMACOLOGY IN ADULTS
- CLINICAL PHARMACOLOGY IN PEDIATRIC PATIENTS
- FAMOTIDINE INDICATIONS AND USAGE
- FAMOTIDINE CONTRAINDICATIONS
- PRECAUTIONS
- FAMOTIDINE ADVERSE REACTIONS
- OVERDOSAGE
- FAMOTIDINE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Principal Display Panel_label
FULL PRESCRIBING INFORMATION
FAMOTIDINE DESCRIPTION
The active ingredient in famotidine for oral suspension is a histamine H2-receptor antagonist. Famotidine is N1-(aminosulfonyl)-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio]propanimidamide. The molecular formula of famotidine is C8H15N7O2S3 and its molecular weight is 337.43. Its structural formula is:
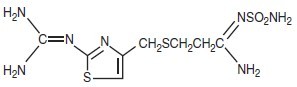
CLINICAL PHARMACOLOGY IN ADULTS
GI Effects
2
Other Effects
ADVERSE REACTIONS4
Pharmacokinetics
PRECAUTIONS DOSAGE AND ADMINISTRATION
PRECAUTIONSGeriatric Use
Clinical Studies
Duodenal Ulcer
| Outpatients with Endoscopically Confirmed Healed Duodenal Ulcers |
|||
|---|---|---|---|
|
|
|
|
|
|
|
FAMOTIDINE 40 mg h.s. (N = 89) |
FAMOTIDINE 20 mg b.i.d. (N = 84) |
Placebo h.s. (N = 97) |
| Week 2 |
**32% |
**38% |
17% |
| Week 4 |
**70% |
**67% |
31% |
Long-Term Maintenance
Treatment of Duodenal Ulcers
Gastric Ulcer
| Patients with Endoscopically Confirmed Healed Gastric Ulcers |
||||
|---|---|---|---|---|
|
|
U.S. Study |
|
International Study |
|
|
|
FAMOTIDINE 40 mg h.s. (N=74) |
Placebo h.s. (N=75) |
FAMOTIDINE 40 mg h.s. (N=149) |
Placebo h.s. (N=145) |
|
|
|
|
|
|
| Week 4 |
45% |
39% |
†47% |
31% |
| Week 6 |
†66% |
44% |
†65% |
46% |
| Week 8 |
***78% |
64% |
†80% |
54% |
†
Gastroesophageal Reflux Disease (GERD)
| % Successful Symptomatic Outcome | |||
|---|---|---|---|
|
|
|
|
|
|
|
FAMOTIDINE 20 mg b.i.d. (N=154) |
FAMOTIDINE 40 mg h.s. (N=149) |
Placebo (N=73) |
| Week 6 |
82††
|
69 |
62 |
††
| % Endoscopic Healing - U.S. Study | |||
|---|---|---|---|
|
|
|
|
|
|
|
FAMOTIDINE 40 mg b.i.d. (N=127) |
FAMOTIDINE 20 mg b.i.d. (N=125) |
Placebo (N=66) |
| Week 6 |
48†††,** ** |
32 |
18 |
| Week 12 |
69†††,* * |
54†††
|
29 |
†††
| % Endoscopic Healing - International Study | |||
|---|---|---|---|
|
|
|
|
|
|
|
FAMOTIDINE 40 mg b.i.d. (N=175) |
FAMOTIDINE 20 mg b.i.d. (N=93) |
Ranitidine 150 mg b.i.d. (N=172) |
| Week 6 |
48 |
52 |
42 |
| Week 12 |
71*** *** |
68 |
60 |
CLINICAL PHARMACOLOGY IN PEDIATRIC PATIENTS
Pharmacokinetics
| Pharmacokinetic Parametersa of Intravenous Famotidine | ||||
|---|---|---|---|---|
|
|
|
|
|
|
| Age (N=number of patients) |
Area Under the Curve (AUC) (ng-hr/mL) |
Total Clearance (Cl) (L/hr/kg) |
Volume of Distribution (Vd) (L/kg) |
Elimination Half-life (T1/2) (hours) |
|
|
|
|
|
|
| 0 to 1 monthc
(N=10) |
NA |
0.13 ± 0.06 |
1.4 ± 0.4 |
10.5 ± 5.4 |
| 0 to 3 monthsd
(N=6) |
2688 ± 847 |
0.21 ± 0.06 |
1.8 ± 0.3 |
8.1 ± 3.5 |
| greater than 3 to 12 monthsd
(N=11) |
1160 ± 474 |
0.49 ± 0.17 |
2.3 ± 0.7 |
4.5 ± 1.1 |
| 1 to 11 yrs (N=20) |
1089 ± 834 |
0.54 ± 0.34 |
2.07 ± 1.49 |
3.38 ± 2.60 |
| 11 to 15 yrs (N=6) |
1140 ± 320 |
0.48 ± 0.14 |
1.5 ± 0.4 |
2.3 ± 0.4 |
| Adult (N=16) |
1726b
|
0.39 ± 0.14 |
1.3 ± 0.2 |
2.83 ± 0.99 |
b
c
d
Pharmacodynamics
max
| Pharmacodynamics of famotidine using the sigmoid E max model | |
|---|---|
|
|
|
|
|
EC50 (ng/mL)* |
| Pediatric Patients |
|
| Data from one study |
26 ± 13 |
| a) healthy adult subjects |
26.5 ± 10.3 |
| b) adult patients with upper GI bleeding |
18.7 ± 10.8 |
| Dosage | Route | Effect
a
|
Number of Patients (age range) |
|
|
|
|
|
| 0.5 mg/kg, single dose |
I.V. |
gastric pH greater than 4 for 19.5 hours (17.3, 21.8)c |
11 (5 to 19 days) |
| 0.3 mg/kg, single dose |
I.V. | gastric pH greater than 3.5 for 8.7 ± 4.7b hours |
6 (2 to 7 years) |
| 0.4 to 0.8 mg/kg |
I.V. | gastric pH greater than 4 for 6-9 hours |
18 (2 to 69 months) |
| 0.5 mg/kg, single dose |
I.V. | a greater than 2 pH unit increase above baseline in gastric pH for greater than 8 hours |
9 (2 to 13 years) |
| 0.5 mg/kg b.i.d. |
I.V. | gastric pH greater than 5 for 13.5 ± 1.8b hours |
4 (6 to 15 years) |
| 0.5 mg/kg b.i.d. |
oral |
gastric pH greater than 5 for 5.0 ± 1.1b hours |
4 (11 to 15 years) |
b
c
FAMOTIDINE INDICATIONS AND USAGE
Famotidine for oral suspension is indicated in:
1. Short-term treatment of active duodenal ulcer. Most adult patients heal within 4 weeks; there is rarely reason to use famotidine at full dosage for longer than 6 to 8 weeks. Studies have not assessed the safety of famotidine in uncomplicated active duodenal ulcer for periods of more than eight weeks.
2. Maintenance therapy for duodenal ulcer patients at reduced dosage after healing of an active ulcer. Controlled studies in adults have not extended beyond one year.
3. Short-term treatment of active benign gastric ulcer. Most adult patients heal within 6 weeks. Studies have not assessed the safety or efficacy of famotidine in uncomplicated active benign gastric ulcer for periods of more than 8 weeks.
4. Short-term treatment of gastroesophageal reflux disease (GERD). Famotidine for oral suspension is indicated for short-term treatment of patients with symptoms of GERD (see CLINICAL PHARMACOLOGY IN ADULTS, Clinical Studies). Famotidine for oral suspension is also indicated for the short-term treatment of esophagitis due to GERD including erosive or ulcerative disease diagnosed by endoscopy (see CLINICAL PHARMACOLOGY IN ADULTS, Clinical Studies).
5. Treatment of pathological hypersecretory conditions (e.g., Zollinger-Ellison Syndrome, multiple endocrine adenomas) (see CLINICAL PHARMACOLOGY IN ADULTS, Clinical Studies).
FAMOTIDINE CONTRAINDICATIONS
2
PRECAUTIONS
General
Patients with Moderate or Severe Renal Insufficiency
CLINICAL PHARMACOLOGY IN ADULTS DOSAGE AND ADMINISTRATION
Information for Patients
Drug Interactions
in vitro
Carcinogenesis, Mutagenesis, Impairment of Fertility
Salmonella typhimuriumEscherichia coliin vivo
Pregnancy
Pregnancy Category B
Nursing Mothers
Pediatric Patients less than 1 year of age
CLINICAL PHARMACOLOGY IN PEDIATRIC PATIENTSPharmacokinetics and Pharmacodynamics
ADVERSE REACTIONSPediatric Patients
Pediatric Patients 1 to 16 years of age
Peptic ulcer
Gastroesophageal Reflux Disease with or without esophagitis including erosions and ulcerations
Geriatric Use
CLINICAL PHARMACOLOGY IN ADULTSPharmacokinetics PRECAUTIONSPatients with Moderate or Severe Renal Insufficiency DOSAGE AND ADMINISTRATIONDosage Adjustment for Patients with Moderate or Severe Renal Insufficiency
FAMOTIDINE ADVERSE REACTIONS
Body as a Whole:
Cardiovascular:
Gastrointestinal:
Hematologic:
Hypersensitivity:
Musculoskeletal:
Nervous System/Psychiatric:
Respiratory:
Skin:
Special Senses:
Other:
Pediatric Patients
OVERDOSAGE
ADVERSE REACTIONS
5050
FAMOTIDINE DOSAGE AND ADMINISTRATION
Duodenal Ulcer
Acute Therapy:
Maintenance Therapy:
Benign Gastric Ulcer
Acute Therapy:
Gastroesophageal Reflux Disease (GERD)
CLINICAL PHARMACOLOGY IN ADULTSClinical Studies
Dosage for Pediatric Patients less than 1 year of age Gastroesophageal Reflux Disease (GERD)
PRECAUTIONSPediatric Patients less than 1 year of age
PRECAUTIONSPediatric Patients less than 1 year of ageGastroesophageal Reflux Disease (GERD)
Dosage for Pediatric Patients 1 to 16 years of age
PRECAUTIONSPediatric Patients 1 to 16 years of age
PRECAUTIONSPediatric Patients 1 to 16 years of age
Peptic ulcer
Gastroesophageal Reflux Disease with or without esophagitis including erosions and ulcerations
Pathological Hypersecretory Conditions (e.g., Zollinger-Ellison Syndrome, Multiple Endocrine Adenomas)
Oral Suspension
Directions for Preparing Famotidine for Oral Suspension
Stability of Famotidine for Oral Suspension
Concomitant Use of Antacids
Dosage Adjustment for Patients with Moderate or Severe Renal Insufficiency
HOW SUPPLIED
Storage
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Distributed by:
Zydus Pharmaceuticals USA Inc.
Principal Display Panel_label
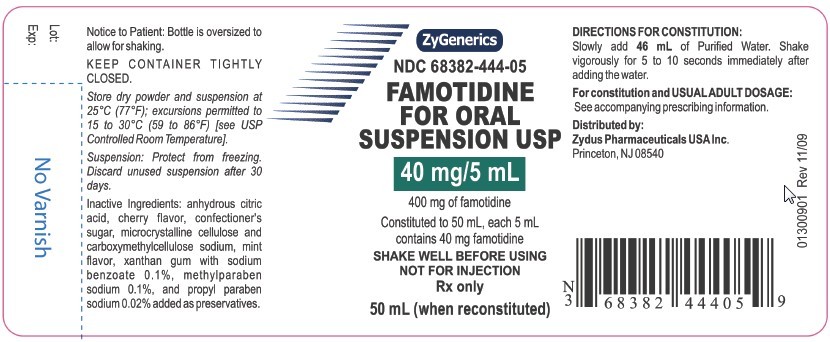
NDC 68382-444-05
FAMOTIDINE FOR ORAL SUSPENSION USP
40 mg/5 mL
400 mg of famotidine
Constituted to 50 mL, each 5 mL contains 40 mg famotidine
SHAKE WELL BEFORE USING NOT FOR INJECTION
Rx Only
50 mL (when reconstituted)
Notice to Patient: Bottle is oversized to allow for shaking.
KEEP CONTAINER TIGHTLY CLOSED.
Store dry powder and suspension at 25°C (77°F); excursions permitted to 15 to 30°C (59 to 86°F) [see USP Controlled Room Temperature].
Suspension: Protect from freezing. Discard unused suspension after 30 days.
Inactive ingredients: anhydrous citric acid, cherry flavor, confectioner’s sugar, microcrystalline cellulose and carboxymethylcellulose sodium, mint
flavor, xanthan gum with sodium benzoate 0.1%, methylparaben sodium 0.1 %, and propylparaben sodium 0.02% added as preservatives.
DIRECTIONS FOR CONSTITUTION:
Slowly add 46 mL of Purified Water. Shake vigorously for 5 to 10 seconds immediately after adding the water.
For constitution and USUAL ADULT DOSAGE: See accompanying prescribing information.
Distributed by:
Zydus Pharmaceuticals USA Inc.
Princeton, NJ 08540
FamotidineFamotidine POWDER, FOR SUSPENSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||