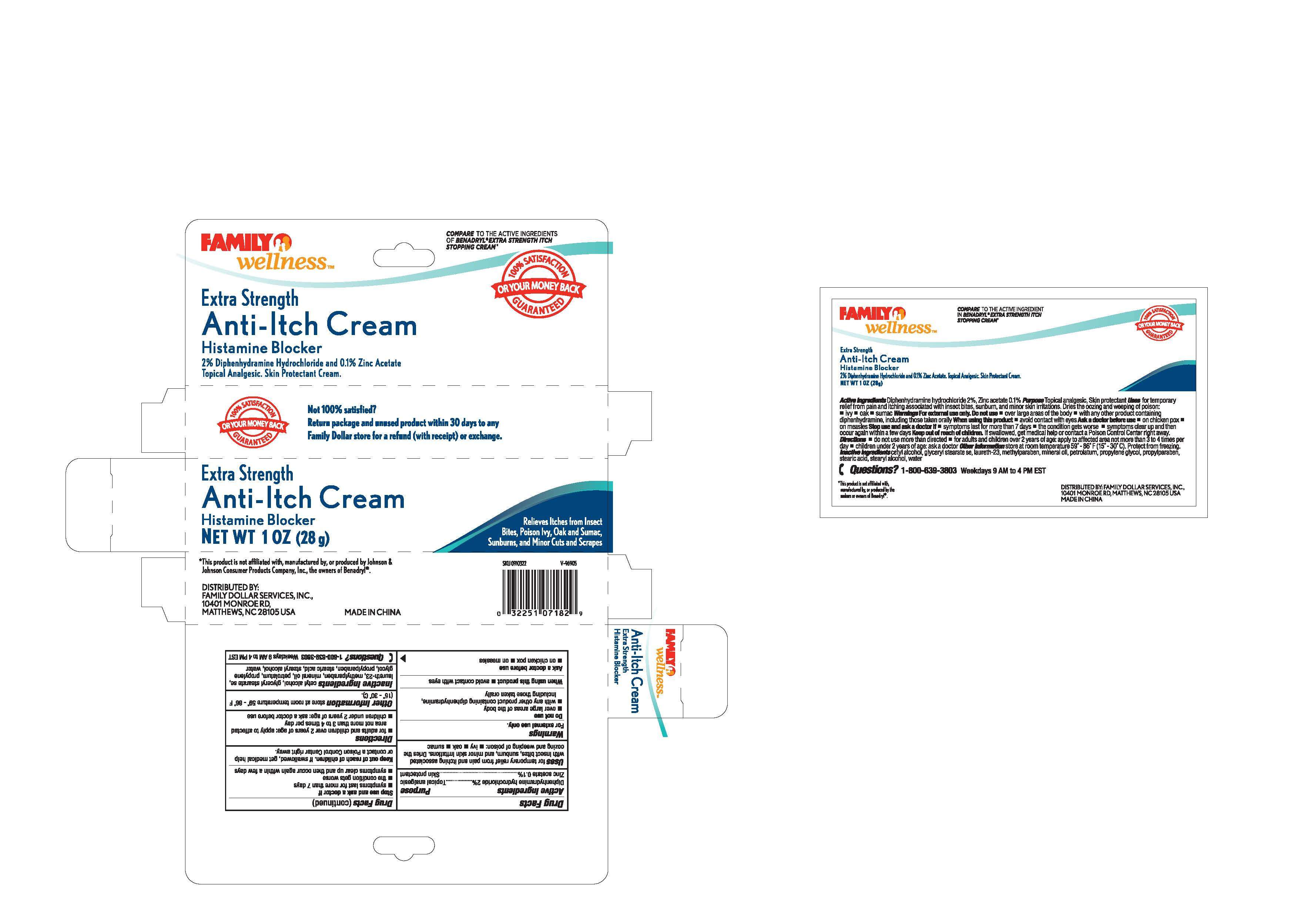
Family Wellness
Zhejiang Jingwei Pharmaceutical Co., Ltd.
Drug Facts
FULL PRESCRIBING INFORMATION
Inactive Ingredients - cetyl alcohol, glycerol stearate,
laureth-23, methylparaben, mineral oil, petrolatum, propylene
glycol, propylparaben, stearic acid, stearyl alcohol, water
Active ingredient
Active Ingredients
Diphenhydramine hydrochloride 2 %
Zinc acetate 0.1 %
Purpose
Purpose
---Topical analgesic
---Skin protectant
Uses
Uses for temporary relief from pain and itching associated
with insect bites, sunburn and minor skin irritations. Dries the
oozing and weeping of poison: -ivy -oak - sumac
Warnings
For external use only
Do not use
-over large areas of the body
-with any other product containing diphenhydramine,
including those taken orally
When using this product - avoid contact with eyes
Ask a doctor before use
-on chicken pox
-on measles
Stop use and ask a doctor if:
-symptoms last for more than 7 days
-the condition gets worse
-symptoms clear up and then occur again within a few days
Keep out of reach of children. If swallowed, get medical help
or contact a Poison Control Center right away.
Directions
-for adults and children over 2 years of age: apply to affected area
not more than 3 or 4 times per day
-children under 2 years of age: ask a doctor before use
Other information - store at room temperature 59 - 86 F (15 - 30 C)
Questions? 1-800-639-3803 Weekdays 9 AM to 4 PM EST
Family WellnessDIPHENHYDRAMINE HYDROCHLORIDE, ZINC ACETATE CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||