FACTIVE
FACTIVE (gemifloxacin mesylate) Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING:
- FACTIVE DESCRIPTION
- CLINICAL PHARMACOLOGY
- FACTIVE INDICATIONS AND USAGE
- FACTIVE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- FACTIVE ADVERSE REACTIONS
- OVERDOSAGE
- FACTIVE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- ANIMAL PHARMACOLOGY
- CLINICAL STUDIES
- REFERENCES
- What is the most important information I should know about FACTIVE?
- What is FACTIVE?
- Who should not take FACTIVE?
- What should I tell my healthcare provider before taking FACTIVE?
- How should I take FACTIVE?
- What should I avoid while taking FACTIVE?
- What are the possible side effects of FACTIVE?
- General Information about FACTIVE
FULL PRESCRIBING INFORMATION
WARNING:
Fluoroquinolones, including FACTIVE®, are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart and lung transplants (See WARNINGS).
To reduce the development of drug-resistant bacteria and maintain the effectiveness of FACTIVE and other antibacterial drugs, FACTIVE should be used only to treat infections that are proven or strongly suspected to be caused by bacteria.
FACTIVE DESCRIPTION
FACTIVE (gemifloxacin mesylate) is a synthetic broad-spectrum antibacterial agent for oral administration. Gemifloxacin, a compound related to the fluoroquinolone class of antibiotics, is available as the mesylate salt in the sesquihydrate form. Chemically, gemifloxacin is (R,S)-7-[(4Z)-3-(aminomethyl)-4-(methoxyimino)-1-pyrrolidinyl]-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid.
The mesylate salt is a white to light brown solid with a molecular weight of 485.49. Gemifloxacin is considered freely soluble at neutral pH (350 μg/mL at 37ºC, pH 7.0). Its empirical formula is C18H20FN5O4•CH4O3S and its chemical structure is:
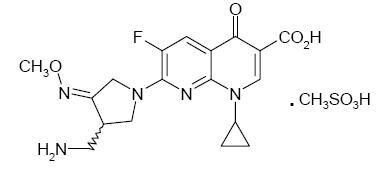
Each white to off-white, oval, film-coated FACTIVE tablet has breaklines and GE 320 debossed on both faces and contains gemifloxacin mesylate equivalent to 320 mg gemifloxacin. The inactive ingredients are crospovidone, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, and titanium dioxide.
CLINICAL PHARMACOLOGY
FACTIVE INDICATIONS AND USAGE
FACTIVE is indicated for the treatment of infections caused by susceptible strains of the designated microorganisms in the conditions listed below. (See DOSAGE AND ADMINISTRATION and CLINICAL STUDIES.)
Acute bacterial exacerbation of chronic bronchitis caused by Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, or Moraxella catarrhalis.
Community-acquired pneumonia (of mild to moderate severity) caused by Streptococcus pneumoniae (including multi-drug resistant strains [MDRSP])*, Haemophilus influenzae, Moraxella catarrhalis, Mycoplasma pneumoniae, Chlamydia pneumoniae, or Klebsiella pneumoniae.
*MDRSP: multi-drug resistant Streptococcus pneumoniae, includes isolates previously known as PRSP (penicillin-resistant Streptococcus pneumoniae), and are strains resistant to two or more of the following antibiotics: penicillin (MIC ≥2 μg/mL), 2nd generation cephalosporins (e.g., cefuroxime), macrolides, tetracyclines and trimethoprim/sulfamethoxazole.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of FACTIVE and other antibacterial drugs, FACTIVE should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
FACTIVE CONTRAINDICATIONS
FACTIVE is contraindicated in patients with a history of hypersensitivity to gemifloxacin, fluoroquinolone antibiotic agents, or any of the product components.
WARNINGS
Tendinopathy and Tendon Rupture: Fluoroquinolones, including FACTIVE, are associated with an increased risk of tendinitis and tendon rupture in all ages. This adverse reaction most frequently involves the Achilles tendon, and rupture of the Achilles tendon may require surgical repair. Tendinitis and tendon rupture in the rotator cuff (the shoulder), the hand, the biceps, the thumb, and other tendon sites have also been reported. The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is further increased in older patients usually over 60 years of age, in those taking corticosteroid drugs, and in patients with kidney, heart or lung transplants. Factors, in addition to age and corticosteroid use, that may independently increase the risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have also occurred in patients taking fluoroquinolones who do not have the above risk factors. Tendon rupture can occur during or after completion of therapy; cases occurring up to several months after completion of therapy have been reported. FACTIVE should be discontinued if the patient experiences pain, swelling, inflammation or rupture of a tendon. Patients should be advised to rest at the first sign of tendinitis or tendon rupture, and to contact their healthcare provider regarding changing to a non-quinolone antimicrobial drug.
THE SAFETY AND EFFECTIVENESS OF FACTIVE IN CHILDREN, ADOLESCENTS (LESS THAN 18 YEARS OF AGE), PREGNANT WOMEN, AND LACTATING WOMEN HAVE NOT BEEN ESTABLISHED. (See PRECAUTIONS: Pediatric Use, Pregnancy and Nursing Mothers subsections.)
QT Effects: Fluoroquinolones may prolong the QT interval in some patients. FACTIVE should be avoided in patients with a history of prolongation of the QTc interval, patients with uncorrected electrolyte disorders (hypokalemia or hypomagnesemia), and patients receiving Class IA (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic agents.
Pharmacokinetic studies between gemifloxacin and drugs that prolong the QTc interval such as erythromycin, antipsychotics, and tricyclic antidepressants have not been performed. FACTIVE should be used with caution when given concurrently with these drugs, as well as in patients with ongoing proarrhythmic conditions, such as clinically significant bradycardia or acute myocardial ischemia. No cardiovascular morbidity or mortality attributable to QTc prolongation occurred with FACTIVE treatment in over 8119 patients, including 707 patients concurrently receiving drugs known to prolong the QTc interval and 7 patients with hypokalemia.
The likelihood of QTc prolongation may increase with increasing dose of the drug; therefore, the recommended dose should not be exceeded especially in patients with renal or hepatic impairment where the Cmax and AUC are slightly higher. QTc prolongation may lead to an increased risk for ventricular arrhythmias including torsades de pointes. The maximal change in the QTc interval occurs approximately 5-10 hours following oral administration of gemifloxacin.
Hypersensitivity Reactions: Serious hypersensitivity and/or anaphylactic reactions have been reported in patients receiving fluoroquinolone therapy, including FACTIVE. Hypersensitivity reactions reported in patients receiving fluoroquinolone therapy have occasionally been fatal. These reactions may occur following the first dose. Some reactions have been accompanied by cardiovascular collapse, hypotension/shock, seizure, loss of consciousness, tingling, angioedema (including tongue, laryngeal, throat or facial edema/swelling), airway obstruction (including bronchospasm, shortness of breath and acute respiratory distress), dyspnea, urticaria, itching and other serious skin reactions.
FACTIVE should be discontinued immediately at the appearance of any sign of an immediate type I hypersensitivity skin rash or any other manifestation of a hypersensitivity reaction; the need for continued fluoroquinolone therapy should be evaluated. As with other drugs, serious acute hypersensitivity reactions may require treatment with epinephrine and other resuscitative measures, including oxygen, intravenous fluids, antihistamines, corticosteroids, pressor amines and airway management as clinically indicated. (See PRECAUTIONS and ADVERSE REACTIONS.)
Other serious and sometimes fatal events, some due to hypersensitivity and some due to uncertain etiology, have been reported rarely in patients receiving therapy with quinolones, including FACTIVE. These events may be severe and generally occur following the administration of multiple doses. Clinical manifestations may include one or more of the following:
fever, rash or severe dermatologic reactions (e.g., toxic epidermal necrolysis, Stevens-Johnson Syndrome);
vasculitis; arthralgia; myalgia; serum sickness;
allergic pneumonitis;
interstitial nephritis; acute renal insufficiency or failure;
hepatitis; jaundice; acute hepatic necrosis or failure;
anemia, including hemolytic and aplastic;
thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia agranulocytosis; pancytopenia; and/or other hematologic abnormalities.
The drug should be discontinued immediately at the first appearance of a skin rash, jaundice, or any other sign of hypersensitivity and supportive measures instituted (See PRECAUTIONS: Information for Patients and ADVERSE REACTIONS).
Peripheral Neuropathy: Rare cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias and weakness have been reported in patients receiving quinolones.
CNS Effects: In clinical studies with FACTIVE, central nervous system (CNS) effects have been reported infrequently. As with other fluoroquinolones, FACTIVE should be used with caution in patients with CNS diseases such as epilepsy or patients predisposed to convulsions. Although not seen in FACTIVE clinical trials, convulsions, increased intracranial pressure, and toxic psychosis have been reported in patients receiving other fluoroquinolones. CNS stimulation which may lead to tremors, restlessness, anxiety, lightheadedness, confusion, hallucinations, paranoia, depression, insomnia, and rarely suicidal thoughts or acts may also be caused by other fluoroquinolones. If these reactions occur in patients receiving FACTIVE, the drug should be discontinued and appropriate measures instituted.
Clostridium difficile Associated Diarrhea: Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including FACTIVE, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
PRECAUTIONS
Prescribing FACTIVE in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Information for Patients
Patients should be counseled:
to contact their healthcare provider if they experience pain, swelling, or inflammation of a tendon, or weakness or inability to use one of their joints; rest and refrain from exercise; and discontinue FACTIVE treatment. The risk of serious tendon disorders with fluoroquinolones is higher in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart and lung transplants;
that antibacterial drugs including FACTIVE should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When FACTIVE is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by FACTIVE or other antibacterial drugs in the future;
that FACTIVE has been associated with rash and hives. Rash occurs more commonly in those under 40, especially women and in women on hormone replacement therapy. The incidence of rash increases with duration more than 5 days and particularly longer than 7 days. Patients should discontinue FACTIVE and call their healthcare provider if they develop a rash;
that FACTIVE may be associated with hypersensitivity reactions, including anaphylactic reactions, even following a single dose; patients should immediately discontinue the drug at the sign of a rash or other allergic reaction and seek medical care;
that diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible;
that FACTIVE may cause changes in the electrocardiogram (QTc interval prolongation);
that FACTIVE should be avoided in patients receiving Class IA (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic agents;
that FACTIVE should be used with caution in patients receiving drugs that affect the QTc interval such as cisapride, erythromycin, antipsychotics, and tricyclic antidepressants;
to inform their physician of any personal or family history of QTc prolongation or proarrhythmic conditions such as hypokalemia, bradycardia, or recent myocardial ischemia;
to contact their physician if they experience palpitations or fainting spells while taking FACTIVE;
that FACTIVE may cause dizziness; if this occurs, patients should not operate an automobile or machinery or engage in activities requiring mental alertness or coordination;
that convulsions have been reported in patients receiving quinolones. Patients should notify their physician before taking FACTIVE if they have a history of convulsions, seizures, or epilepsy;
that other central nervous system problems such as tremors, restlessness, lightheadedness, confusion and hallucinations may occur rarely;
that photosensitivity/phototoxicity has been reported in patients receiving quinolones. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while taking quinolones. If patients need to be outdoors while using quinolones, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. If a sunburn-like reaction or skin eruption occurs, patients should contact their physician; (See CLINICAL PHARMACOLOGY: Photosensitivity Potential);
that increases of the International Normalized Ratio (INR), or prothrombin time (PT), and/or clinical episodes of bleeding have been noted with concurrent administration of warfarin or its derivatives, and FACTIVE. Patients should notify their physicians if they are taking warfarin or its derivatives;
to inform their physician of any other medications when taken concurrently with FACTIVE, including over-the-counter medications and dietary supplements;
that FACTIVE may be taken with or without meals;
to drink fluids liberally;
not to take antacids containing magnesium and/or aluminum or products containing ferrous sulfate (iron), multivitamin preparations containing zinc or other metal cations, or Videx® (didanosine) chewable/buffered tablets or the pediatric powder for oral solution within 3 hours before or 2 hours after taking FACTIVE tablets;
that FACTIVE should be taken at least 2 hours before sucralfate.
Drug Interactions:
Administration of repeat doses of FACTIVE had no effect on the repeat dose pharmacokinetics of theophylline, digoxin or an ethinylestradiol/levonorgestrol oral contraceptive product in healthy subjects. (See CLINICAL PHARMACOLOGY: Drug-Drug Interactions.)
Concomitant administration of FACTIVE and calcium carbonate, cimetidine, omeprazole, or an estrogen/progesterone oral contraceptive produced minor changes in the pharmacokinetics of gemifloxacin, which were considered to be without clinical significance. (See CLINICAL PHARMACOLOGY.)
Concomitant administration of FACTIVE with probenecid resulted in a 45% increase in systemic exposure to gemifloxacin. (See CLINICAL PHARMACOLOGY.)
FACTIVE had no significant effect on the anticoagulant effect of warfarin in healthy subjects on stable warfarin therapy. However, post-marketing reports of increases in the INR, or PT, and/or clinical episodes of bleeding in patients have been noted with the use of quinolones, including FACTIVE, and warfarin, or its derivatives. In addition, infectious disease and its accompanying inflammatory process, age and general status of the patient are risk factors for increased anticoagulation activity. Therefore, the PT, INR or other suitable coagulation test should be closely monitored if a quinolone antimicrobial, including FACTIVE, is administered concomitantly with warfarin or its derivatives.
Quinolones form chelates with alkaline earth and transition metals. The absorption of oral gemifloxacin is significantly reduced by the concomitant administration of an antacid containing aluminum and magnesium. Magnesium- and/or aluminum-containing antacids, products containing ferrous sulfate (iron), multivitamin preparations containing zinc or other metal cations, or Videx® (didanosine) chewable/buffered tablets or the pediatric powder for oral solution should not be taken within 3 hours before or 2 hours after FACTIVE. Sucralfate should not be taken within 2 hours of FACTIVE. (See CLINICAL PHARMACOLOGY.)
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Long term studies in animals to determine the carcinogenic potential of gemifloxacin have not been conducted.
Photocarcinogenesis: Gemifloxacin did not shorten the time to development of UVR-induced skin tumors in hairless albino (Skh-1) mice; thus, it was not photocarcinogenic in this model. These mice received oral gemifloxacin and concurrent irradiation with simulated sunlight 5 days per week for 40 weeks followed by a 12-week treatment-free observation period. The daily dose of UV radiation used in this study was approximately 1/3 of the minimal dose of UV radiation that would induce erythema in Caucasian humans. The median time to the development of skin tumors in the hairless mice was similar in the vehicle control group (36 weeks) and those given up to 100 mg/kg gemifloxacin daily (39 weeks). Following repeat doses of 100 mg/kg gemifloxacin per day, the mice had skin gemifloxacin concentrations of approximately 7.4 μg/g. Plasma levels following this dose were approximately 1.4 μg/mL in the mice around the time of irradiation. There are no data on gemifloxacin skin levels in humans, but the mouse plasma gemifloxacin levels are in the expected range of human plasma Cmax levels (0.7-2.6 μg/mL, with an overall mean of about 1.6 μg/mL) following multiple 320 mg oral doses.
Mutagenesis: Gemifloxacin was not mutagenic in 4 bacterial strains (TA 98, TA 100, TA 1535, TA 1537) used in an Ames Salmonella reversion assay. It did not induce micronuclei in the bone marrow of mice following intraperitoneal doses of up to 40 mg/kg and it did not induce unscheduled DNA synthesis in hepatocytes from rats which received oral doses of up to 1600 mg/kg. Gemifloxacin was clastogenic in vitro in the mouse lymphoma and human lymphocyte chromosome aberration assays. It was clastogenic in vivo in the rat micronucleus assay at oral and intravenous dose levels (≥800 mg/kg and ≥40 mg/kg, respectively) that produced bone marrow toxicity. Fluoroquinolone clastogenicity is apparently due to inhibition of mammalian topoisomerase activity which has threshold implications.
Impairment of Fertility: Gemifloxacin did not affect the fertility of male or female rats at AUC levels following oral administration (216 and 600 mg/kg/day) that were approximately 3- to 4-fold higher than the AUC levels at the clinically recommended dose.
Pregnancy:
Teratogenic Effects.
Pregnancy Category C. Gemifloxacin treatment during organogenesis caused fetal growth retardation in mice (oral dosing at 450 mg/kg/day), rats (oral dosing at 600 mg/kg/day) and rabbits (IV dosing at 40 mg/kg/day) at AUC levels which were 2-, 4- and 3-fold those in women given oral doses of 320 mg. In rats, this growth retardation appeared to be reversible in a pre- and postnatal development study (mice and rabbits were not studied for the reversibility of this effect). Treatment of pregnant rats at 8-fold clinical exposure (based upon AUC comparisons) caused fetal brain and ocular malformations in the presence of maternal toxicity. The overall no-effect exposure level in pregnant animals was approximately 0.8 to 3-fold clinical exposure.
The safety of FACTIVE in pregnant women has not been established. FACTIVE should not be used in pregnant women unless the potential benefit to the mother outweighs the risk to the fetus. There are no adequate and well-controlled studies in pregnant women.
Nursing Mothers:
Gemifloxacin is excreted in the breast milk of rats. There is no information on excretion of gemifloxacin into human milk. Therefore, FACTIVE should not be used in lactating women unless the potential benefit to the mother outweighs the risk.
Pediatric Use:
Safety and effectiveness in children and adolescents less than 18 years of age have not been established. Fluoroquinolones, including gemifloxacin, cause arthropathy and osteochondrosis in immature animals. (See WARNINGS.)
Geriatric Use:
Geriatric patients are at increased risk for developing severe tendon disorders including tendon rupture when being treated with a fluoroquinolone such as FACTIVE. This risk is further increased in patients receiving concomitant corticosteroid therapy. Tendinitis or tendon rupture can involve the Achilles, hand, shoulder, or other tendon sites and can occur during or after completion of therapy; cases occurring up to several months after fluoroquinolone treatment have been reported. Caution should be used when prescribing FACTIVE to elderly patients especially those on corticosteroids. Patients should be informed of this potential side effect and advised to discontinue FACTIVE and contact their healthcare provider if any symptoms of tendinitis or tendon rupture occur (See Boxed Warning, WARNINGS, and ADVERSE REACTIONS/Post-Marketing Adverse Event Reports).
Of the total number of subjects in clinical studies of FACTIVE, 29% (2314) were 65 and over, while 11% (865) were 75 and over. No overall difference in effectiveness was observed between these subjects and younger subjects; the adverse event rate for this group was similar to or lower than that for younger subjects with the exception that the incidence of rash was lower in geriatric patients compared to patients less than 40 years of age.
Elderly patients may be more susceptible to drug-associated effects on the QT interval. Therefore, FACTIVE should be avoided in patients taking drugs that can result in prolongation of the QT interval (e.g., Class IA or Class III antiarrhythmics) or in patients with risk factors for torsades de pointes (e.g., known QT prolongation, uncorrected hypokalemia).
FACTIVE ADVERSE REACTIONS
In clinical studies, 8119 patients received daily oral doses of 320 mg FACTIVE. In addition, 1797 healthy volunteers and 81 patients with renal or hepatic impairment received single or repeat doses of gemifloxacin in clinical pharmacology studies. The majority of adverse reactions experienced by patients in clinical trials were considered to be of mild to moderate severity.
FACTIVE was discontinued because of an adverse event (determined by the investigator to be possibly or probably related to drug) in 2.0% of patients, primarily due to rash (0.8%), nausea (0.3%), diarrhea (0.3%), urticaria (0.2%) and vomiting (0.2%). Comparator antibiotics were discontinued because of an adverse event at an overall comparable rate of 2.1%, primarily due to diarrhea (0.5%), nausea (0.4%), vomiting (0.3%), rash (0.3%), abdominal pain (0.2%) and vertigo (0.2%).
The most commonly reported adverse events with a frequency of ≥2% for patients receiving 320 mg FACTIVE versus comparator drug (beta-lactam antibiotics, macrolides or other fluoroquinolones) are as follows: diarrhea 5.0% vs. 6.2%; rash 3.5% vs. 1.1%; nausea 3.7% vs. 4.5%; headache 4.2% vs. 5.2%; abdominal pain 2.2% vs. 2.2%; vomiting 1.6% vs. 2.0%; and dizziness 1.7% vs. 2.6%.
Adverse Events with a Frequency of Less than 1%
Additional drug-related adverse events (possibly or probably related) in the 8119 patients, with a frequency of >0.1% to ≤1% included: abdominal pain, anorexia, constipation, dermatitis, dizziness, dry mouth, dyspepsia, fatigue, flatulence, fungal infection, gastritis, genital moniliasis, genital pruritus, hyperglycemia, increased alkaline phosphatase, increased ALT, increased AST, increased creatine phosphokinase, insomnia, leukopenia, pruritus, somnolence, taste perversion, thrombocythemia, urticaria, vaginitis, and vomiting.
Other adverse events reported from clinical trials which have potential clinical significance and which were considered to have a suspected relationship to the drug, that occurred in ≤0.1% of patients were: abnormal urine, abnormal vision, anemia, arthralgia, asthenia, back pain, bilirubinemia, dyspnea, eczema, eosinophilia, facial edema, flushing, gastroenteritis, granulocytopenia, hot flashes, increased GGT, increased non-protein nitrogen, leg cramps, moniliasis, myalgia, nervousness, non-specified gastrointestinal disorder, pain, pharyngitis, photosensitivity/phototoxicity reactions, pneumonia, thrombocytopenia, tremor, vertigo. (See PRECAUTIONS.)
In clinical trials of acute bacterial exacerbation of chronic bronchitis (ABECB) and community acquired pneumonia (CAP), the incidences of rash were as follows (Table 3):
| ABECB (5 days) N = 2284 | CAP (5 days) N = 256 | CAP (7 days) N = 643 | ||||
| n/N | % | n/N | % | n/N | % | |
| * insufficient number of patients in this category for a meaningful analysis | ||||||
| Totals | 27/2284 | 1.2 | 1/256 | 0.4 | 26/643 | 4.0 |
| Females, < 40 years | NA* | 1/37 | 2.7 | 8/88 | 9.1 | |
| Females, ≥ 40 years | 16/1040 | 1.5 | 0/73 | 0 | 5/214 | 2.3 |
| Males, < 40 years | NA* | 0/65 | 0 | 5/101 | 5.0 | |
| Males, ≥ 40 years | 11/1203 | 0.9 | 0/81 | 0 | 8/240 | 3.3 |
(See PRECAUTIONS).
Laboratory Changes:
The percentages of patients who received multiple doses of FACTIVE and had a laboratory abnormality are listed below. It is not known whether these abnormalities were related to FACTIVE or an underlying condition.
Clinical Chemistry: increased ALT (1.7%), increased AST (1.3%), increased creatine phosphokinase (0.7%), increased alkaline phosphatase (0.4%), increased total bilirubin (0.4%), increased potassium (0.3%), decreased sodium (0.2%), increased blood urea nitrogen (0.3%), decreased albumin (0.3%), increased serum creatinine (0.2%), decreased calcium (0.1%), decreased total protein (0.1%), decreased potassium (0.1%), increased sodium (0.1%), increased lactate dehydrogenase (<0.1%) and increased calcium (<0.1%).
CPK elevations were noted infrequently: 0.7% in FACTIVE patients vs. 0.7% in the comparator patients.
Hematology: increased platelets (1.0%), decreased neutrophils (0.5%), increased neutrophils (0.5%), decreased hematocrit (0.3%), decreased hemoglobin (0.2%), decreased platelets (0.2%), decreased red blood cells (0.1%), increased hematocrit (0.1%), increased hemoglobin (0.1%), and increased red blood cells (0.1%).
In clinical studies, approximately 7% of the FACTIVE treated patients had elevated ALT values immediately prior to entry into the study. Of these patients, approximately 15% showed a further elevation of their ALT at the on-therapy visit and 9% showed a further elevation at the end of therapy visit. None of these patients demonstrated evidence of hepatocellular jaundice. For the pooled comparators, approximately 6% of patients had elevated ALT values immediately prior to entry into the study. Of these patients, approximately 7% showed a further elevation of their ALT at the on-therapy visit and 4% showed a further elevation at the end of therapy visit.
In a clinical trial where 638 patients received either a single 640 mg dose of gemifloxacin or 250 mg BID of ciprofloxacin for 3 days, there was an increased incidence of ALT elevations in the gemifloxacin arm (3.9%) vs. the comparator arm (1.0%). In this study, two patients experienced ALT elevations of 8 to 10 times the upper limit of normal. These elevations were asymptomatic and reversible.
Post-Marketing Side Effects:
The majority of the post-marketing adverse events reported were cutaneous and most of these were rash. Some of these cutaneous adverse events were considered serious. The majority of the rashes occurred in women and in patients under 40 years of age.
The following are additional adverse reactions reported during the post-marketing use of FACTIVE. Since these reactions are reported voluntarily from a population of uncertain size, it is impossible to reliably estimate their frequency or establish a causal relationship to FACTIVE exposure:
anaphylactic reaction, erythema multiforme, skin exfoliation, facial swelling;
hemorrhage, increased international normalized ratio (INR), retinal hemorrhage;
peripheral edema;
renal failure;
prolonged QT, supraventricular tachycardia, syncope, transient ischemic attack;
photosensitivity/phototoxicity reaction (See PRECAUTIONS.);
antibiotic-associated colitis;
tendon rupture.
OVERDOSAGE
Any signs or symptoms of overdosage should be treated symptomatically. No specific antidote is known. In the event of acute oral overdosage, the stomach should be emptied by inducing vomiting or by gastric lavage; the patient should be carefully observed and treated symptomatically with appropriate hydration maintained. Hemodialysis removes approximately 20 to 30% of an oral dose of gemifloxacin from plasma.
Mortality occurred at oral gemifloxacin doses of 1600 mg/kg in rats and 320 mg/kg in mice. The minimum lethal intravenous doses in these species were 160 and 80 mg/kg, respectively. Toxic signs after administration of a single high oral dose (400 mg/kg) of gemifloxacin to rodents included ataxia, lethargy, piloerection, tremor, and clonic convulsions.
FACTIVE DOSAGE AND ADMINISTRATION
FACTIVE can be taken with or without food and should be swallowed whole with a liberal amount of liquid. The recommended dose of FACTIVE is 320 mg daily, according to the following table (Table 4).
The clinical decision regarding the use of a 5 or 7 day regimen should be guided by results of the initial sputum culture.
| INDICATION | DOSE / DURATION |
| *MDRSP: multi-drug resistant Streptococcus pneumoniae, includes isolates previously known as PRSP (penicillin-resistant Streptococcus pneumoniae), and are strains resistant to two or more of the following antibiotics: penicillin (MIC ≥2 µg/mL), 2nd generation cephalosporins (e.g., cefuroxime), macrolides, tetracyclines and trimethoprim/sulfamethoxazole. | |
| Acute bacterial exacerbation of chronic | One 320 mg tablet daily for 5 days |
| bronchitis | |
| Community-acquired pneumonia (of mild to moderate severity) | |
| due to known or suspected S. pneumoniae, | |
| H. influenzae, M. pneumoniae, or C. | One 320 mg tablet daily for 5 days |
| pneumoniae infection | |
| due to known or suspected MDRSP*, K. | One 320 mg tablet daily for 7 days |
| pneumoniae, or M. catarrhalis infection | |
The recommended dose and duration of FACTIVE should not be exceeded (see Table 2).
Use in Renally Impaired Patients: Dose adjustment in patients with creatinine clearance >40 mL/min is not required. Modification of the dosage is recommended for patients with creatinine clearance ≤40 mL/min. Table 5 provides dosage guidelines for use in patients with renal impairment.
| Creatinine Clearance (mL/min) | Dose |
| >40 | See Usual Dosage |
| ≤40 | 160 mg every 24 hours |
Patients requiring routine hemodialysis or continuous ambulatory peritoneal dialysis (CAPD) should receive 160 mg every 24 hours.
When only the serum creatinine concentration is known, the following formula may be used to estimate creatinine clearance.

Women: 0.85 x the value calculated for men
Use in Hepatically Impaired Patients: No dosage adjustment is recommended in patients with mild (Child-Pugh Class A), moderate (Child-Pugh Class B) or severe (Child-Pugh Class C) hepatic impairment.
Use in Elderly: No dosage adjustment is recommended.
HOW SUPPLIED
FACTIVE (gemifloxacin mesylate) is available as white to off-white, oval, film-coated tablets with breaklines and GE 320 debossed on both faces. Each tablet contains gemifloxacin mesylate equivalent to 320 mg of gemifloxacin.
320 mg Unit of Use (CR*) 5's NDC 67707-320-05
320 mg Unit of Use (CR*) 7's NDC 67707-320-07
*Child Resistant
Storage
Store at 25ºC (77ºF); excursions permitted to 15º-30ºC (59º-86ºF) [see USP Controlled Room Temperature]. Protect from light.
ANIMAL PHARMACOLOGY
Quinolones have been shown to cause arthropathy in immature animals. Degeneration of articular cartilage occurred in juvenile dogs given at least 192 mg/kg/day gemifloxacin in a 28-day study (producing about 6 times the systemic exposure at the clinical dose), but not in mature dogs. There was no damage to the articular surfaces of joints in immature rats given repeated doses of up to 800 mg/kg/day.
Some quinolones have been reported to have proconvulsant properties that are potentiated by the concomitant administration of non-steroidal anti-inflammatory drugs (NSAIDs). Gemifloxacin alone had effects in tests of behavior or CNS interaction typically at doses of at least 160 mg/kg. No convulsions occurred in mice given the active metabolite of the NSAID, fenbufen, followed by 80 mg/kg gemifloxacin.
Dogs given 192 mg/kg/day (about 6 times the systemic exposure at the clinical dose) for 28 days, or 24 mg/kg/day (approximately equivalent to the systemic exposure at the clinical dose) for 13 weeks showed reversible increases in plasma ALT activities and local periportal liver changes associated with blockage of small bile ducts by crystals containing gemifloxacin.
Quinolones have been associated with prolongation of the electrocardiographic QT interval in dogs. Gemifloxacin produced no effect on the QT interval in dogs dosed orally to provide about 4 times human therapeutic plasma concentrations at Cmax, and transient prolongation after intravenous administration at more than 4 times human plasma levels at Cmax. Gemifloxacin exhibited weak activity in the cardiac IKr (hERG) channel inhibition assay, having an IC50 of approximately 270 μM.
Gemifloxacin, like many other quinolones, tends to crystallize at the alkaline pH of rodent urine, resulting in a nephropathy in rats that is reversible on drug withdrawal (oral no-effect dose 24 mg/kg/day).
Gemifloxacin was weakly phototoxic to hairless mice given a single 200 mg/kg oral dose and exposed to UVA radiation. However, no evidence of phototoxicity was observed at 100 mg/kg/day dosed orally for 13 weeks in a standard hairless mouse model, using simulated sunlight.
CLINICAL STUDIES
Acute Bacterial Exacerbation of Chronic Bronchitis (ABECB)
FACTIVE (320 mg once daily for 5 days) was evaluated for the treatment of acute bacterial exacerbation of chronic bronchitis in three pivotal double-blind, randomized, actively-controlled clinical trials (studies 068, 070, and 212). The primary efficacy parameter in these studies was the clinical response at follow-up (day 13 to 24). The results of the clinical response at follow-up for the principal ABECB studies demonstrate that FACTIVE 320 mg PO once daily for 5 days was at least as good as the comparators given for 7 days. The results are shown in Table 6 below.
| Drug Regimen | Success Rate % (n/N) | Treatment Difference (95% CI) |
| Study 068 | ||
| FACTIVE 320 mg x 5 days | 86.0 (239/278) | 1.2 (-4.7, 7.0) |
| Clarithromycin 500 mg BID x 7 days | 84.8 (240/283) | |
| Study 070 | ||
| FACTIVE 320 mg x 5 days | 93.6 (247/264) | 0.4 (-3.9, 4.6) |
| Amoxicillin/clavulanate 500 mg/125 mg TID x 7 days | 93.2 (248/266) | |
| Study 212 | ||
| FACTIVE 320 mg x 5 days | 88.2 (134/152) | 3.1 (-4.7, 10.7) |
| Levofloxacin 500 mg x 7 days | 85.1 (126/148) | |
Community Acquired Pneumonia (CAP)
5 Day Treatment Regimen
To evaluate the safety and efficacy of a 5-day course of FACTIVE, 510 outpatient and hospitalized adults with clinically and radiologically determined mild to moderate community-acquired pneumonia were clinically evaluated in a double-blind, randomized, prospective, multicenter study comparing FACTIVE 320 mg for five days to FACTIVE 320 mg for seven days (Study OP-634-001).
Clinical success rates in the clinically evaluable population were 95.0% in the 5 day group and 92.1% in the 7 day group.
| Drug Regimen | Success Rate % (n/N) | Treatment Difference (95% CI) |
| Study OP-634-001 | ||
| FACTIVE 320 mg x 5 days | 95.0 (230/242) | 3.0 (-1.5, 7.4) |
| FACTIVE 320 mg x 7 days | 92.1 (209/227) | |
The microbiological efficacy of the 5-day regimen was documented for pathogens listed in Table 8 below.
| 5-day | 7-day | |||
| Pathogen | n/N | % | n/N | % |
| Streptococcus pneumoniae | 26/26 | 100 | 34/40 | 85.0 |
| Mycoplasma pneumoniae | 22/25 | 88.0 | 19/20 | 95.0 |
| Haemophilus influenzae | 21/22 | 95.5 | 18/18 | 100 |
| Chlamydia pneumoniae | 17/18 | 94.4 | 30/31 | 96.8 |
7 Day Treatment Regimen
Previous clinical studies evaluated the efficacy of FACTIVE in a 7-day treatment of CAP in adults. This clinical program consisted of three double-blind, randomized, actively-controlled clinical studies (studies 011, 012, and 049) and one open-label, actively-controlled study (study 185). In addition, two uncontrolled studies (studies 061 and 287) were conducted. Three of the studies, controlled study 011 and the uncontrolled studies, had a fixed 7-day duration of treatment for FACTIVE. Controlled study 011 compared a 7-day course of FACTIVE with a 10-day treatment course of amoxicillin/clavulanate (1g/125 mg TID) and clinical success rates were similar between treatment arms. The results of comparative studies 049, 185, and 012 were supportive although treatment duration could have been 7 to 14 days. The results of the clinical studies with a fixed 7-day duration of FACTIVE are shown in Table 9.
| Drug Regimen | Success Rate % (n/N) | Treatment Difference (95% CI)* |
| *For uncontrolled studies, the 95% CI around the success rate is shown | ||
| Study 011 | ||
| FACTIVE 320 mg x 7 days | 88.7 (102/115) | 1.1 (-7.3, 9.5) |
| Amoxicillin/clavulanate 1 g/125 mg TID x 10 days | 87.6 (99/113) | |
| Study 061 | ||
| FACTIVE 320 mg x 7 days | 91.7 (154/168) | (86.1, 95.2) |
| Study 287 | ||
| FACTIVE 320 mg x 7 days | 89.8 (132/147) | (84.9, 94.7) |
The combined bacterial eradication rates for patients treated with a fixed 7-day treatment regimen of FACTIVE are shown in Table 10.
| Pathogen | n/N | % |
| S. pneumoniae | 102/117 | 87.2 |
| M. pneumoniae | 40/42 | 95.2 |
| H. influenzae | 48/53 | 90.6 |
| C. pneumoniae | 43/45 | 95.6 |
| K. pneumoniae | 18/20 | 90.0 |
| M. catarrhalis | 11/12 | 91.7 |
7 Day Treatment Regimen of Community-Acquired Pneumonia Due to Multi-Drug Resistant Streptococcus pneumoniae (MDRSP)
FACTIVE was also effective in the treatment of CAP due to multi-drug resistant Streptococcus pneumoniae (MDRSP*). Of 35 patients with MDRSP treated for 7 days, 29 (82.9%) achieved clinical and bacteriological success at follow-up. The clinical and bacteriological success for the 35 patients with MDRSP isolates are shown in Table 11.
*MDRSP: multi-drug resistant Streptococcus pneumoniae, includes isolates previously known as PRSP (penicillin-resistant Streptococcus pneumoniae), and are strains resistant to two or more of the following antibiotics: penicillin (MIC ≥2 μg/mL), 2nd generation cephalosporins (e.g., cefuroxime), macrolides, tetracyclines and trimethoprim/sulfamethoxazole.
| Screening Susceptibility | Clinical Success | Bacteriological Success | ||
| n/Na | % | n/Nb | % | |
| an = the number of patients successfully treated; N = number of patients with MDRSP | ||||
| bn = the number of bacteriological isolates successfully treated; N = number of isolates studied | ||||
| cMacrolide antibiotics tested include clarithromycin and erythromycin | ||||
| Penicillin-resistant | 15/16 | 93.8 | 15/16 | 93.8 |
| 2nd generation cephalosporin-resistant | 20/22 | 90.9 | 20/22 | 90.9 |
| Macrolide-resistantc | 24/28 | 85.7 | 23/28 | 82.1 |
| Trimethoprim/sulfamethoxazole-resistant | 23/26 | 88.5 | 23/26 | 88.5 |
| Tetracycline-resistant | 21/27 | 77.8 | 20/27 | 74.1 |
Not all isolates were resistant to all antimicrobial classes tested. Success and eradication rates are summarized in Table 12 below.
| S. pneumoniae with MDRSP | Clinical Cure Rate | Bacteriological Eradication Rate | ||
| n/N | % | n/N | % | |
| Resistant to 2 antimicrobials | 8/11 | 72.7 | 7/11 | 63.6 |
| Resistant to 3 antimicrobials | 5/7 | 71.4 | 5/7 | 71.4 |
| Resistant to 4 antimicrobials | 8/9 | 88.9 | 8/9 | 88.9 |
| Resistant to 5 antimicrobials | 8/8 | 100 | 8/8 | 100 |
| Bacteremia with MDRSP | 3/3 | 100 | 3/3 | 100 |
Clinical Safety Study of Rash
To further characterize gemifloxacin-associated rash, which in early clinical studies appeared to be associated with age less than 40 and female gender, a clinical pharmacology study was conducted. The study enrolled 1,011 healthy female volunteers less than 40 years of age. Subjects were randomized in a 5:1 ratio to receive either FACTIVE 320 mg PO daily (819 subjects) or ciprofloxacin 500 mg PO twice daily for 10 days (164 subjects). This study was designed to enroll subjects at a high risk for rash (women <40 years of age and dosing beyond the recommended duration of therapy for FACTIVE [10 days]) and over estimates the risk to patients taking FACTIVE as prescribed. Subjects who received FACTIVE were 7 times more likely to develop rash than those who received ciprofloxacin. Of the 260 rashes in subjects receiving FACTIVE, the majority of rashes were maculopapular and of mild to moderate severity; 7% of the rashes were reported as severe, and severity appeared to correlate with the extent of the rash. In 68% of the subjects reporting a severe rash and approximately 25% of all those reporting rash, >60% of the body surface area was involved; the characteristics of the rash were otherwise indistinguishable from those subjects reporting a mild rash. The histopathology was consistent with the clinical observation of uncomplicated exanthematous morbilliform eruption. Approximately 11% of the rashes were described as being “urticaria-like”. There were no documented cases of hypersensitivity syndrome or findings suggestive of angioedema or other serious cutaneous reactions.
The majority of rashes (81.9%) occurred on days 8 through 10 of the planned 10 day course of FACTIVE; 2.7% of rash events occurred within one day of the start of dosing. The median duration of rash was 6 days. The rash resolved without treatment in the majority of subjects. Approximately 19% received antihistamines and 5% received steroids, although the therapeutic benefit of these therapies is uncertain.
In the second part of this study after a 4 to 6 week wash out period, subjects developing a rash on FACTIVE were treated with ciprofloxacin (n=136) or placebo (n=50); 5.9% developed rash when treated with ciprofloxacin and 2.0% developed rash when treated with placebo. The cross sensitization rate to other fluoroquinolones was not evaluated in this clinical study. There was no evidence of sub-clinical sensitization to FACTIVE on a second exposure (i.e., subjects who had not developed a rash to FACTIVE in the first part of the study were not at higher risk of developing a rash to FACTIVE with a second exposure).
There was no relationship between the incidence of rash and systemic exposure (Cmax and AUC) to either gemifloxacin or its major metabolite, N-acetyl gemifloxacin.
REFERENCES
1. Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically-Seventh Edition. Clinical and Laboratory Standards Institute document M7-A7, Vol. 26, No. 2, CLSI, Wayne, PA, January 2006.
2. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests-Ninth Edition. Clinical and Laboratory Standards Institute document M2-A9, Vol. 26, No. 1, CLSI, Wayne, PA, January 2006.
DATE OF REVISION October 2008
© Oscient Pharmaceuticals Corporation 2008
FACTIVE is a registered trademark of LG Life Sciences.
Rx only
Manufactured for:
Oscient Pharmaceuticals
Waltham, MA 02451-1478 USA
Licensed from LG Life Sciences, Ltd. Seoul, Korea
MEDICATION GUIDE
FACTIVE® [FAC-tiv]
(gemifloxacin)
320mg Tablets
Read the Medication Guide that comes with FACTIVE before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about FACTIVE?
FACTIVE belongs to a class of antibiotics called fluoroquinolones. FACTIVE can cause side effects that may be serious or even cause death. If you get any of the following serious side effects, get medical help right away. Talk with your healthcare provider about whether you should continue to take FACTIVE.
Tendon rupture or swelling of the tendon (tendinitis)
Tendons are tough cords of tissue that connect muscles to bones.
Pain, swelling, tears, and inflammation of tendons including the back of the ankle (Achilles), shoulder, hand, or other tendon sites can happen in people of all ages who take fluoroquinolone antibiotics, including FACTIVE. The risk of getting tendon problems is higher if you:
are over 60 years of age
are taking steroids (corticosteroids)
have had a kidney, heart or lung transplant.
Swelling of the tendon (tendinitis) and tendon rupture (breakage) have also happened in patients who take fluoroquinolones who do not have the above risk factors.
Other reasons for tendon ruptures can include:
physical activity or exercise
kidney failure
tendon problems in the past, such as in people with rheumatoid arthritis (RA).
Call your healthcare provider right away at the first sign of tendon pain, swelling or inflammation. Stop taking FACTIVE until tendinitis or tendon rupture has been ruled out by your healthcare provider. Avoid exercise and using the affected area. The most common area of pain and swelling is the Achilles tendon at the back of your ankle. This can also happen with other tendons. Talk to your healthcare provider about the risk of tendon rupture with continued use of FACTIVE. You may need a different antibiotic that is not a fluoroquinolone to treat your infection.
Tendon rupture can happen while you are taking or after you have finished taking FACTIVE. Tendon ruptures have happened up to several months after patients have finished taking their fluoroquinolone.
Get medical help right away if you get any of the following signs or symptoms of a tendon rupture:
hear or feel a snap or pop in a tendon area
bruising right after an injury in a tendon area
unable to move the affected area or bear weight
See the section “What are the possible side effects of FACTIVE?” for more information about side effects.
What is FACTIVE?
FACTIVE is a fluoroquinolone antibiotic medicine used to treat certain types of infections caused by certain germs called bacteria. It is not known if FACTIVE is safe and works in children under 18 years of age. Children have a higher chance of getting bone, joint, or tendon (musculoskeletal) problems such as pain or swelling while taking FACTIVE.
Sometimes infections are caused by viruses rather than by bacteria. Examples include viral infections in the sinuses and lungs, such as the common cold or flu. Antibiotics including FACTIVE do not kill viruses.
Call your healthcare provider if you think your condition is not getting better while you are taking FACTIVE.
Who should not take FACTIVE?
Do not take FACTIVE if you have ever had a severe allergic reaction to an antibiotic known as a fluoroquinolone, or are allergic to any of the ingredients in FACTIVE. Ask your healthcare provider if you are not sure. See the list of ingredients in FACTIVE at the end of this Medication Guide.
What should I tell my healthcare provider before taking FACTIVE?
See “What is the most important information I should know about FACTIVE?”
Tell your healthcare provider about all your medical conditions, including if you:
have tendon problems
have central nervous system problems (such as epilepsy)
have nerve problems
have or anyone in your family has an irregular heartbeat, especially a condition called “QT prolongation”
have low blood potassium (hypokalemia) or magnesium (hypomagnesemia)
have a slow heartbeat (bradycardia)
have a history of seizures
have kidney problems. You may need a lower dose of FACTIVE if your kidneys do not work well.
have rheumatoid arthritis (RA) or other history of joint problems
are pregnant or planning to become pregnant. It is not known if FACTIVE will harm your unborn child.
are breast-feeding or planning to breast-feed. It is not known if FACTIVE passes into breast milk. You and your healthcare provider should decide whether you will take FACTIVE or breast-feed.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal and dietary supplements. FACTIVE and other medicines can affect each other causing side effects. Especially tell your healthcare provider if you take:
an NSAID (Non-Steroidal Anti-Inflammatory Drug). Many common medicines for pain relief are NSAIDs. Taking an NSAID while you take FACTIVE or other fluoroquinolones may increase your risk of central nervous system effects and seizures. See “What are the possible side effects of FACTIVE?”
a blood thinner (warfarin, Coumadin, Jantoven)
a medicine to control your heart rate or rhythm (antiarrhythmics). See “What are the possible side effects of FACTIVE?”
erythromycin
an anti-psychotic medicine
a tricyclic antidepressant
a water pill (diuretic)
probenecid (Probalan, Col-Probenecid)
a steroid medicine. Corticosteroids taken by mouth or by injection may increase the chance of tendon injury. See “What is the most important information I should know about FACTIVE?”
Certain medicines may keep FACTIVE from working correctly. Take FACTIVE either 2 hours before or 3 hours after taking these products:
an antacid, multivitamin, or other product that contains magnesium, aluminum, iron, or zinc.
sucralfate (Carafate).
didanosine (Videx®, Videx® EC).
Ask your healthcare provider if you are not sure if any of your medicines are listed above.
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take FACTIVE?
Take FACTIVE exactly as prescribed by your healthcare provider.
Take FACTIVE at about the same time each day.
FACTIVE can be taken with or without food.
Swallow FACTIVE whole, and drink plenty of fluids with it. Do not chew FACTIVE. Tell your healthcare provider if you are not able to swallow FACTIVE whole. You will need to take a different antibiotic medicine.
Do not skip any doses, or stop taking FACTIVE even if you begin to feel better, until you finish your prescribed treatment, unless:
you have tendon effects (see “What is the most important information I should know about FACTIVE?”),
you have a serious allergic reaction (see “What are the possible side effects of FACTIVE?”), or
your healthcare provider tells you to stop.
This will help make sure that all of the bacteria are killed and lower the chance that the bacteria will become resistant to FACTIVE. If this happens, FACTIVE and other antibiotic medicines may not work in the future.
If you miss a dose of FACTIVE, take it as soon as you remember. Do not take more than 1 dose of FACTIVE in one day.
If you take too much, call your healthcare provider or get medical help immediately.
What should I avoid while taking FACTIVE?
FACTIVE can make you feel dizzy and lightheaded. Do not drive, operate machinery, or do other activities that require mental alertness or coordination until you know how FACTIVE affects you.
Avoid sunlamps, tanning beds, and try to limit your time in the sun. FACTIVE can make your skin sensitive to the sun (photosensitivity) and the light from sunlamps and tanning beds. You could get severe sunburn, blisters or swelling of your skin. If you get any of these symptoms while taking FACTIVE, call your healthcare provider right away. You should use sunscreen and wear a hat and clothes that cover your skin if you have to be in sunlight.
What are the possible side effects of FACTIVE?
FACTIVE can cause side effects that may be serious or even cause death. See “What is the most important information I should know about FACTIVE?”
Other serious side effects of FACTIVE include:
Central Nervous System Effects. Seizures have been reported in people who take fluoroquinolone antibiotics, including FACTIVE. Tell your healthcare provider if you have a history of seizures. Ask your healthcare provider whether taking FACTIVE will change your risk of having a seizure.
Central Nervous System (CNS) side effects may occur as soon as after taking the first dose of FACTIVE. Talk to your healthcare provider right away if you get any of these side effects, or other changes in mood or behavior:feel lightheaded
seizures
hear voices, see things, or sense things that are not there (hallucinations)
feel restless
tremors
feel anxious or nervous
confusion
depression
trouble sleeping
feel more suspicious (paranoia)
suicidal thoughts or acts
nightmares
Serious allergic reactions. Allergic reactions can happen in people taking fluoroquinolones, including FACTIVE, even after only one dose. Stop taking FACTIVE and get emergency medical help right away if you get any of the following symptoms of a severe allergic reaction:
hives
trouble breathing or swallowing
swelling of the lips, tongue, face
throat tightness, hoarseness
rapid heartbeat
fain
yellowing of the skin or eyes. Stop taking FACTIVE and call your healthcare provider right away if you get yellowing of your skin or white part of your eyes, or if you have dark urine. These can be signs of a serious reaction to FACTIVE (a liver problem).
Skin rash. Skin rash may happen in people taking FACTIVE. Stop taking FACTIVE at the first sign of a skin rash and call your healthcare provider. Skin rash may be a sign of a more serious reaction to FACTIVE. Rash happens more often with FACTIVE in:
women, especially women who take hormone replacement therapy
people under 40 years of age
people who take FACTIVE for longer than 5 days.
Serious heart rhythm changes (QTc prolongation and torsades de pointes).
Tell your healthcare provider right away if you have a change in your heartbeat (get a fast or irregular heartbeat), or if you faint. FACTIVE may cause a rare heart problem known as prolongation of the QT interval. This condition can cause an abnormal heartbeat and can be very dangerous. The chances of this happening are higher in people:who are elderly
with a family history of prolonged QT interval
with low blood potassium (hypokalemia)
who take certain medicines to control heart rhythm (antiarrhythmics).
Intestine infection (Pseudomembranous colitis). Pseudomembranous colitis can happen with most antibiotics, including FACTIVE. Call your healthcare provider right away if you get watery diarrhea, diarrhea that does not go away, or bloody stools. You may have stomach cramps and a fever. Pseudomembranous colitis can happen 2 or more months after you have finished your antibiotic.
Changes in sensation and possible nerve damage (Peripheral Neuropathy). Damage to the nerves in arms, hands, legs, or feet can happen in people taking fluoroquinolones, including FACTIVE. Talk with your healthcare provider right away if you get any of the following symptoms of peripheral neuropathy in your arms, hands, legs, or feet:
pain
burning
tingling
numbness
weakness
FACTIVE may need to be stopped to prevent permanent nerve damage.
Sensitivity to sunlight (photosensitivity)
See “What should I avoid while taking FACTIVE?”
The most common side effects of FACTIVE include:
diarrhea
rash
nausea
headache
stomach pain
vomiting
dizziness
These are not all the possible side effects of FACTIVE. Tell your healthcare provider about any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store FACTIVE?
Store FACTIVE at 59º - 86ºF (15º to 30ºC).
Keep FACTIVE away from light.
Keep FACTIVE and all medicines out of the reach of children.
General Information about FACTIVE
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use FACTIVE for a condition for which it is not prescribed. Do not give FACTIVE to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about FACTIVE. If you would like more information about FACTIVE, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about FACTIVE that is written for healthcare professionals. For more information go to www.FACTIVE.com or call 1-866-432-2848.
What are the ingredients in FACTIVE?
Active ingredient: gemifloxacin
Inactive ingredients: crospovidone, hydroxypropyl methycellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, titanium dioxide.
Revised October 2008
© Oscient Pharmaceuticals Corporation 2008
FACTIVE is a registered trademark of LG Life Sciences.
Manufactured for:
Oscient Pharmaceuticals
Waltham, MA 02451-1478 USA
Licensed from LG Life Sciences, Ltd. Seoul, Korea
This Medication Guide has been approved by the U.S. Food and Drug Administration.
FACTIVEgemifloxacin mesylate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||