Face Lift Broad Spectrum SPF 20
Jafra Cosmetics International Inc
Jafra Cosmetics International Inc
Jafra Face Lift Broad Spectrum SPF 20
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active Ingredients Purpose
Avobenzone 3% Sunscreen
Homosalate 5% Sunscreen
Octisalate 5% Sunscreen
Octocrylene 7% Sunscreen
Uses
helps prevent sunburn
If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warning
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Warning
Stop use and ask a doctor if rash occurs
Warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Directions
Apply liberally 15 minutes before sun exposure
Reapply at least every 2 hours
Use a water resistant sunscreen if swimming or sweating
Children under 6 months: Ask a doctor
Sun Protection Measures. Spending time in the sun increases your risk of skin
cancer and early skin aging. To decrease this risk, regularly use a sunscreen
with broad spectrum SPF of 15 or higher and other sun protection measures
including:
limit time in the sun, especially from 10 a.m. - 2 p.m.
wear long-sleeve shirts, pants, hats, and sunglasses
Inactive ingredients
Water/Aqua, Butylene Glycol, Sorbitan Laurate, Hydroxyethylcellulose, Acetyl Dipeptide-1, Cetyl Ester, Pentylene Glycol, Cetearyl Alcohol, Coco-glucoside, Algae Extract, Pullulan, Niacinamide, Hydrolyzed Soy Protein, Zea Mays (Corn) Kernel Extract, Xanthan Gum, Sodium Hyaluronate, Trehalose, Sodium PCA, Polyquaternium-51, Urea, Glycerin, Avena Sativa (Oat) Kernel Extract, Cocos Nucifera (Coconut) Oil, Cucumis Sativus (Cucumber) Fruit Extract, Santalum Album (Sandalwood) Wood Extract, Rose Extract, Pyrus Malus, Hydrolyzed Soy Protein, Zea (Apple) Fruit Extract, Passiflora Incarnata Fruit Extract, Prunus Armeniaca (Apricot) Fruit Extract, Rubus Idaeus (Raspberry) Fruit Extract, Cananga Odorata Flower Extract, Cucumis Melo Cantalupensis Fruit Extract, C20-22 Alkyl Phosphate, C20-22 Alcohols, Caprylic/Capric Triglyceride, Alaria Esculenta Extract, Yeast Amino Acids, Tricalcium Phosphate, Morinda Citrifolia Extract, Carica Papaya Extract, Glycyrrhiza Glabra (Licorice) Extract, Pichia Anomala Extract, Tripleurospermum Maritima Extract, Microcrystalline Cellulose, Cellulose Gum, Dipotassium Glycyrrhizate, Glycine Soja (Soybean) Oil, Arnica Montana Flower Extract, Tocopheryl, Trisodium EDTA, PEG-4 Laurate, Iodopropynyl Butlcarbamate, Menthyl Lactate, Benzylidene Dimethoxydimethylindanone, Polyacrylamide, C13-14 Isoparaffin, Laureth-7.
Principle Display
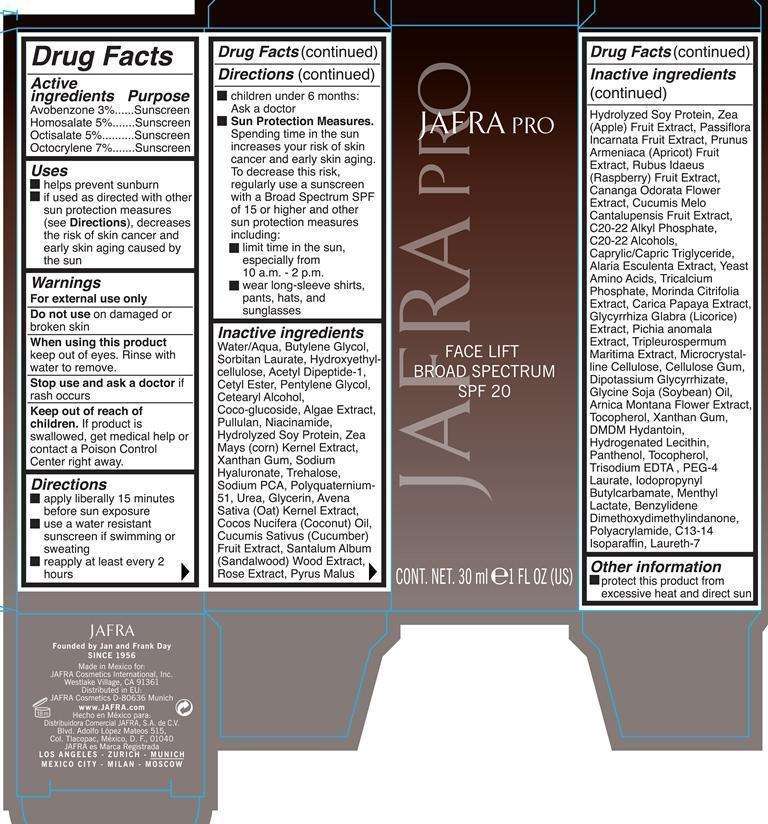
Jafra Pro
Face Lift Broad Spectrum SPF 20
Cont. Net. 30 ml 1 Fl Oz