FACE IT Radiance Two Way Cake NB21
THEFACESHOP CO., LTD
THEFACESHOP CO., LTD
FACE IT Radiance Two Way Cake NB21
FULL PRESCRIBING INFORMATION
Active ingredient
For external use only
Keep out of eyes. Rinse with water to remove.
Stop use if a rash or irritation develops and lasts.
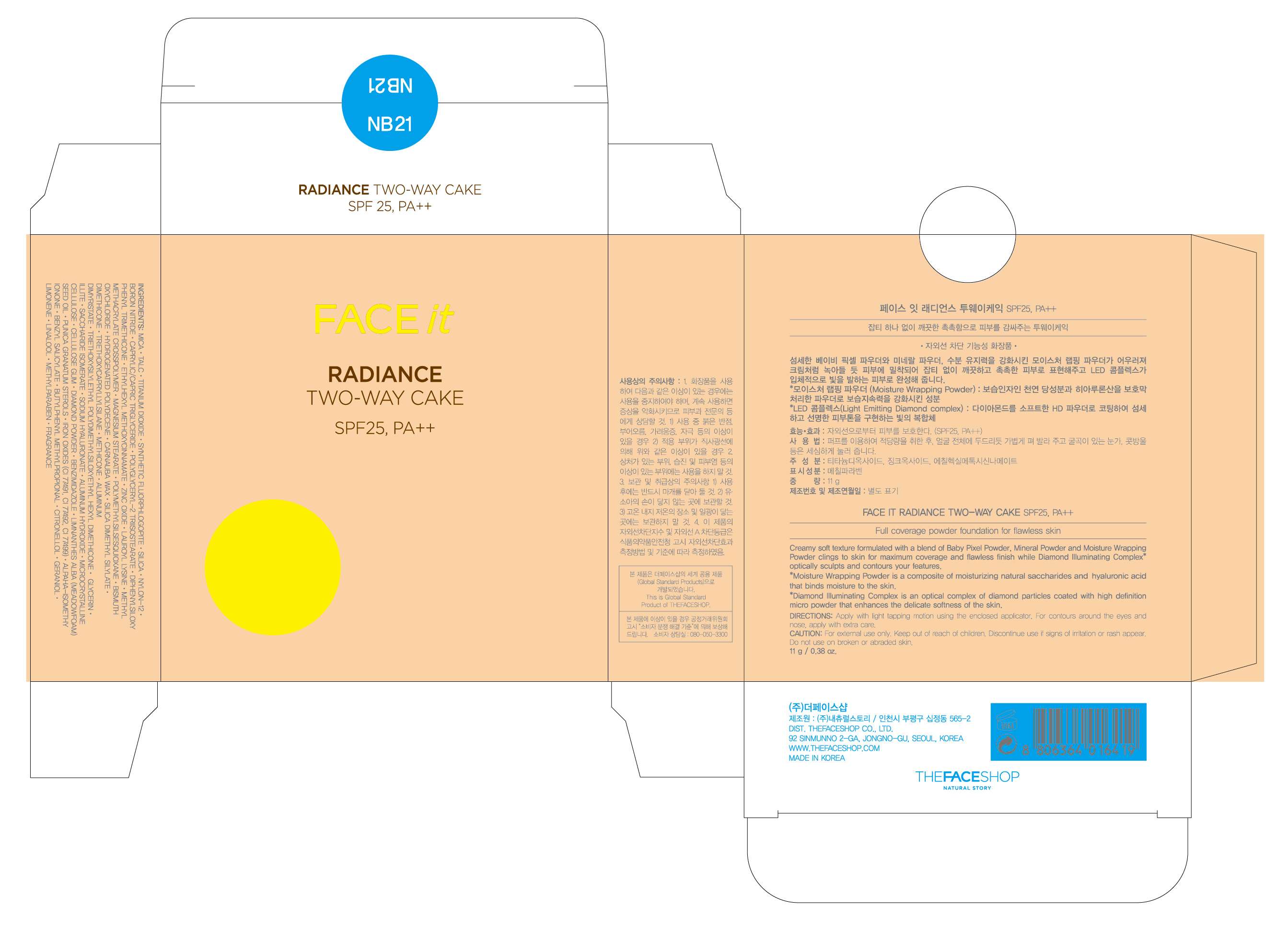
FACE IT Radiance Two Way Cake NB21TITANIUM DIOXIDE, OCTINOXATE, ZINC OXIDE POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!