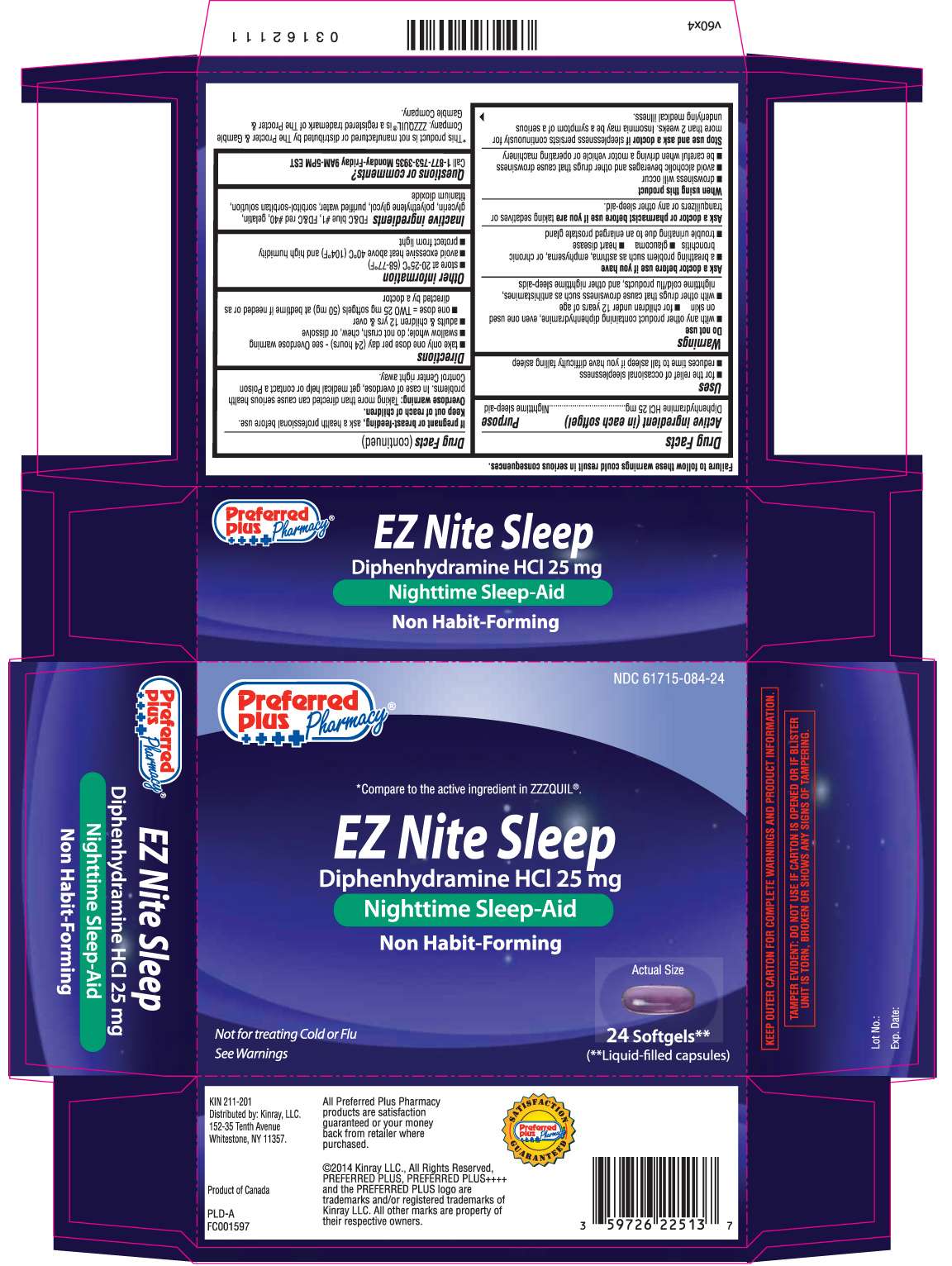
EZ Nite Sleep
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each softgel)
- Purpose
- EZ Nite Sleep Uses
- Warnings
- Directions
- EZ Nite Sleep Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
- Product Label
FULL PRESCRIBING INFORMATION
Active ingredient (in each softgel)
Diphenhydramine HCl 25 mg
Purpose
Nighttime sleep-aid
EZ Nite Sleep Uses
- for the relief of occasional sleeplessness
- reduces time to fall asleep if you have difficulty falling asleep
Warnings
Do not use
- with any other product containing diphenhydramine, even one used on skin
- for children under 12 years of age
- with other drugs that cause drowsiness such as antihistamines, nighttime cold/flu products, and other nighttime sleep-aids
Ask a doctor before use if you have
- a breathing problem such as asthma, emphysema, or chronic bronchitis
- glaucoma
- heart disease
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers or any other sleep-aid.
When using this product
- drowsiness will occur
- avoid alcoholic beverages and other drugs that cause drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
Overdose warning: Taking more than directed can cause serious health problems. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- take only one dose per day (24 hours) - see Overdose warning
- swallow whole; do not crush, chew, or dissolve
- adults & children 12 yrs & over
- one dose=TWO 25 mg softgels (50 mg) at bedtime if needed or as directed by a doctor
EZ Nite Sleep Other information
- store at 20-25°C (68-77°F)
- avoid excessive heat above 40°C (104°F) and high humidity
- protect from light
Inactive ingredients
FD&C blue #1, FD&C red #40, gelatin, glycerin, polyethylene glycol, purified water, sorbitol-sorbitan solution, titanium dioxide
Questions or comments?
Call 1-877-753-3935 Monday- Friday 9AM-5PM EST
Principal Display Panel
Preferred Plus Pharmacy® ++++
*Compare to the active ingredient in ZzzQuil®
EZ Nite Sleep
Diphenhydramine HCl 25 mg
Nighttime Sleep- Aid
Non Habit- forming
NOT FOR TREATING COLD OR FLU
SEE WARNINGS
Softgels**
**Liquid-filled capsules
KIN 211-201
Distributed by: Kinray, LLC
152-35 tenth avenue
Whitestone, NY 11357
Product of Canada
All Preferred Plus Pharmacy products are satisfaction guaranteedor your money back from retailer where purchased.
© 2014 Kinray, LLC., All Rights Reserved
PREFERRED PLUS, PREFERRED PLUS++++ and the PREFERRED PLUS logo are trademarks and/or registered trademarks of Kinray LLC. All other marks are property of their respective owners.
*This product is not manufactured or distributed by Procter & Gamble company, ZzzQuil® is a registered trademark of The Procter & Gamble company.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING.
Product Label
EZ Nite SleepDiphenhydramine HCl CAPSULE, LIQUID FILLED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||