Eyewash Station Additive Concentrate
EYEWASH STATION ADDITIVE CONCENTRATE
FULL PRESCRIBING INFORMATION: CONTENTS*
- Ingredients
- Purpose
- Use
- Warnings
- Directions
- Eyewash Station Additive Concentrate Other information
- Questions ?
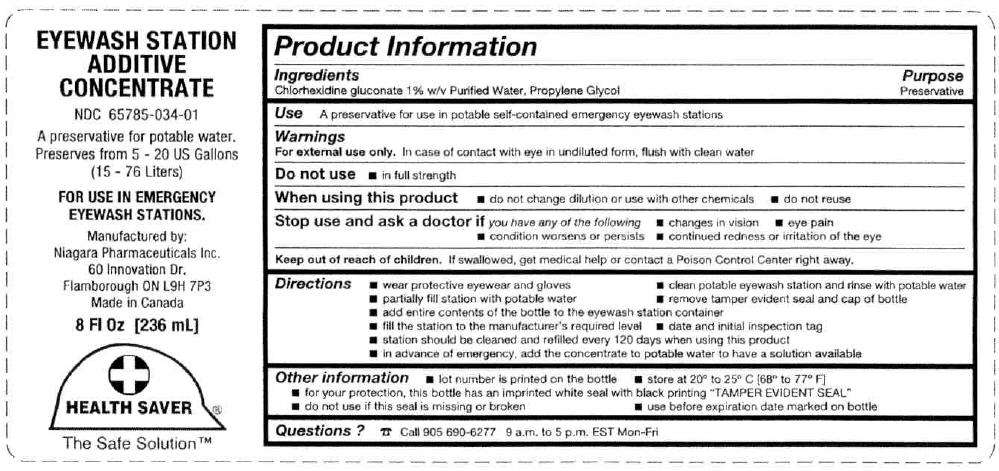
- PRINCIPAL DISPLAY PANEL - 236 mL Bottle Label
FULL PRESCRIBING INFORMATION
Product Information
Ingredients
Chlorhexidine gluconate 1% w/v Purified Water, Propylene Glycol
Purpose
Preservative
Use
A preservative for use in potable self-contained emergency eyewash stations
Warnings
For external use only. In case of contact with eye in undiluted form, flush with clean water
Do not use
- in full strength
When using this product
- do not change dilution or use with other chemicals
- do not reuse
Stop use and ask a doctor if you have any of the following
- changes in vision
- eye pain
- condition worsens or persists
- continued redness or irritation of the eye
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- wear protective eyewear and gloves
- clean potable eyewash station and rinse with potable water
- partially fill station with potable water
- remove tamper evident seal and cap of bottle
- add entire contents of the bottle to the eyewash station container
- fill the station to the manufacturer's required level
- date and initial inspection tag
- station should be cleaned and refilled every 120 days when using this product
- in advance of emergency, add the concentrate to potable water to have a solution available
Eyewash Station Additive Concentrate Other information
- lot number is printed on the bottle
- store at 20° to 25° C [68° to 77° F]
- for your protection, this bottle has an imprinted white seal with black printing "TAMPER EVIDENT SEAL"
- do not use if this seal is missing or broken
- use before expiration date marked on bottle
Questions ?
☎ Call 905 690-6277 9 a.m. to 5 p.m. EST Mon-Fri
Manufactured by:
Niagara Pharmaceuticals Inc.
60 Innovation Dr.
Flamborough ON L9H 7P3
PRINCIPAL DISPLAY PANEL - 236 mL Bottle Label
EYEWASH STATION
ADDITIVE
CONCENTRATE
NDC 65785-034-01
A preservative for potable water.
Preserves from 5 - 20 US Gallons
(15 - 76 Liters)
FOR USE IN EMERGENCY
EYEWASH STATIONS.
Manufactured by:
Niagara Pharmaceuticals Inc.
60 Innovation Dr.
Flamborough ON L9H 7P3
Made in Canada
8 Fl Oz [236 mL]
HEALTH SAVER®
The Safe Solution™
Eyewash Station Additive ConcentrateChlorhexidine gluconate and Propylene Glycol LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||