Eye Wash
MWI/VetOne
Bausch & Lomb Incorporated
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Purified water (99.05%)
Eyewash
washes the eye to help relieve
- irritation
- stinging
- itching
by removing
- loose foreign material
- air pollutants (smog or pollen)
- chlorinated water
Do not use
if solution changes color or becomes cloudy
When using this product
- remove contact lenses before using
- do not touch tip of container to any surface to avoid contamination
- replace cap after use
Stop use and ask a doctor if
- you experience eye pain, changes in vision, continued redness or irritation of the eye
- condition worsens or persists
- you have open wounds in or near the eye
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
- flush the affected eye(s) as needed
- control the rate of flow of solution by pressure on the bottle
When using an eye cup
- rinse the cup with clean water immediately before each use
- avoid contamination of the rim and inside surfaces of the cup
- fill the cup half full with and apply the cup to the affected eye(s), pressing tightly to prevent the escape of the liquid
- tilt the head backward. Open eyelids wide and rotate eyeball to ensure thorough bathing with the wash or lotion
- rinse cup with clean water after each use
- store at 15º–30ºC (59º–86ºF)
- keep tightly closed
Boric acid, sodium borate and sodium chloride. Hydrochloric acid and/or sodium hydroxide may be used to adjust pH. PRESERVATIVE ADDED: edetate disodium 0.025% and sorbic acid 0.1%
Serious side effects associated with use of this product may be reported to: 1-800-323-0000
Package/Label Principal Display Panel
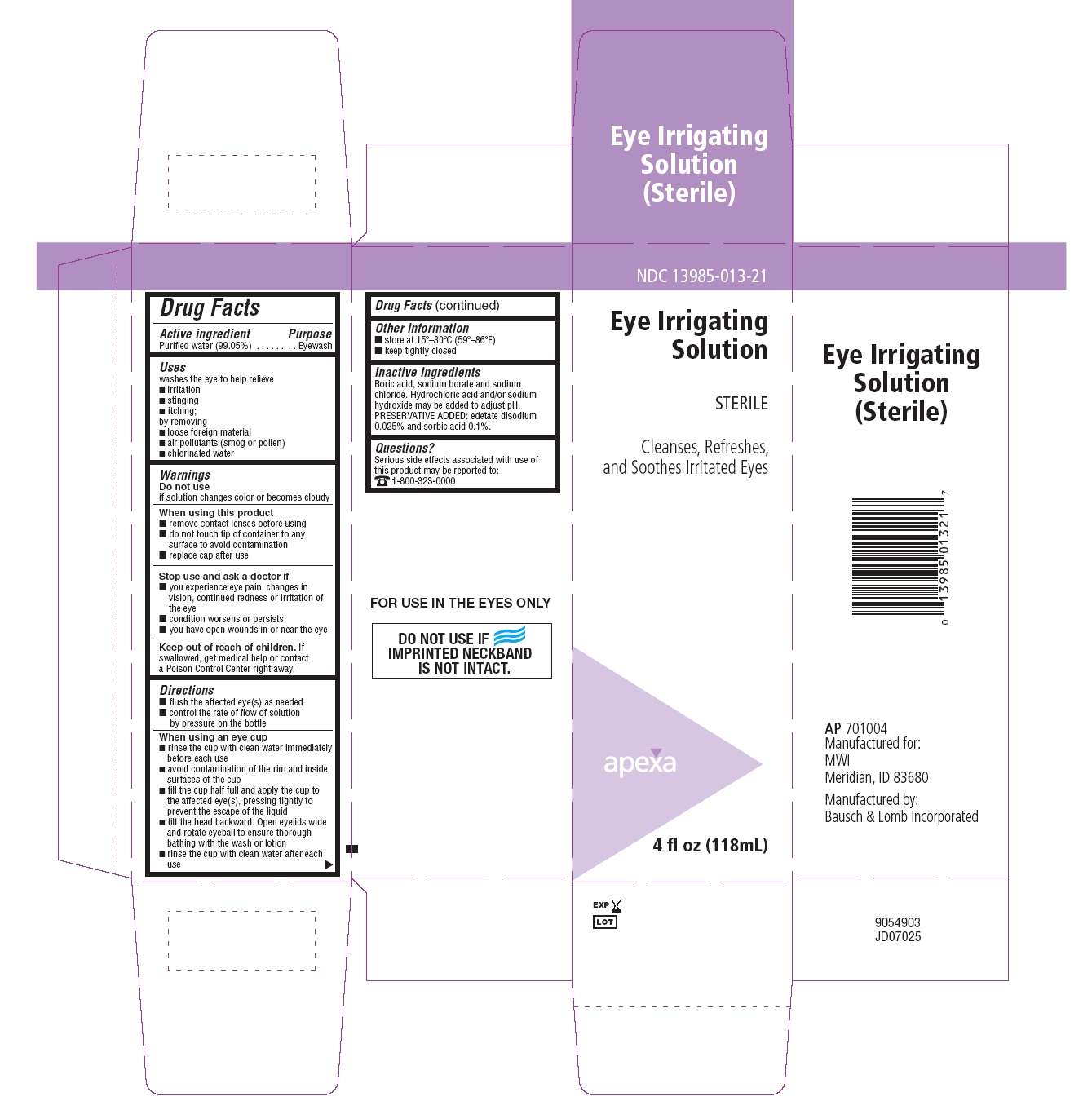
NDC 13985-013-21
Eye Irrigating Solution STERILE
Cleanses, Refreshes, and Soothes Irritated Eyes
apexa
4 fl oz (118 mL)
Eye Washwater SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!