Eye Wash
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Eye Wash Uses
- Warnings
- Directions
- Eye Wash Other information
- Inactive ingredients
- Package label
FULL PRESCRIBING INFORMATION
Active ingredient
Purified water (99.05%)
Purpose
Eyewash
Eye Wash Uses
washes the eye to help relieve
- irritation
- stinging
- discomfort
- itching;
by removing
- loose foreign material
- air pollutants (smog or pollen)
- chlorinated water
Warnings
Do not use
- if you have open wounds in or near the eyes, and get medical help right away
Stop use and ask a doctor if
- you experience eye pain, changes in vision, continued redness or irritation of the eye
- condition worsens or persists
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Remove contact lenses before using.
- Do not touch tip of container to any surface to avoid contamination.
- Replace cap after use.
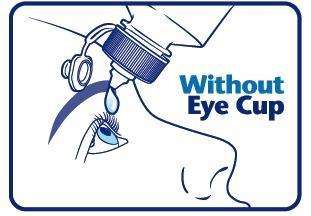

When using an eye cup
- rinse the cup with Pure-aid Eye Wash immediately before each use
- avoid contamination of the rim and inside surfaces of the cup
- fill the cup half full with Pure-aid Eye Wash Solution and apply the cup to the affected eye(s), pressing tightly to prevent spillage
- tilt the head backward. Open eyelids wide and rotate eyeball to thoroughly wash the eye
- rinse cup with clean water after each use
Eye Wash Other information
- store at room temperature
- keep tightly closed
- use before expiration date marked on the carton or bottle
Inactive ingredients
boric acid, sodium borate, sodium chloride, Hydrochloric acid PRESERVATIVE ADDED: edetate disodium, polyhexamethylene biguanide
Package label
Advanced Eye Wash
Eye Washwater SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!