Eye Drops SY
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- Keep out of reach of children.
- Eye Drops SY Uses
- Warnings
- Directions
- Inactive Ingredients
- Eye Drops SY Other information
- Principal Display Panel
FULL PRESCRIBING INFORMATION
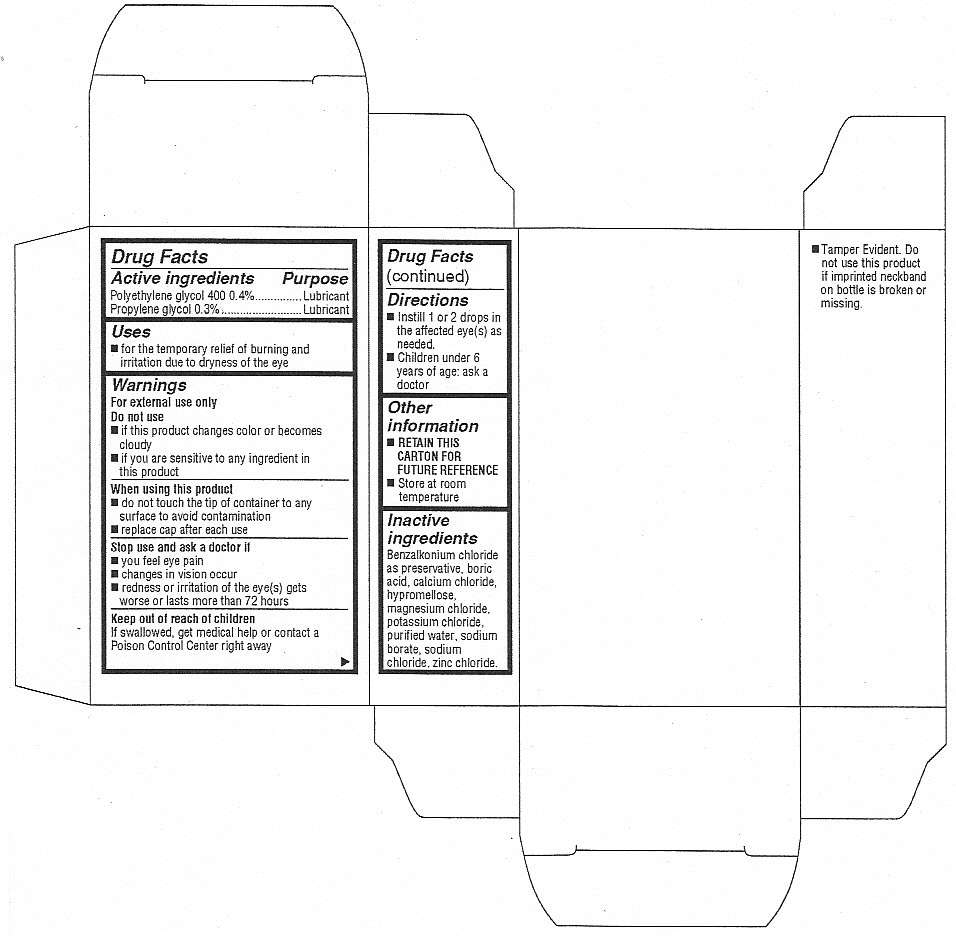
Active Ingredients
Polyethylene glycol 400 0.4%
Propylene glycol 0.3%
Purpose
Polyethylene glycol 400 - Lubricant
Propylene glycol - Lubricant
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Eye Drops SY Uses
- for the temporary relief of burning and irritation due to dryness of the eye
Warnings
For external use only
Do not use
- if this product changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- do not touch the tip of container to any surface to avoid contamination
- replace cap after each use
Stop use and ask a doctor if
- you feel eye pain
- changes in vision occur
- redness or irritation of the eye(s) gets worse or lasts more than 72 hours
Directions
- Instill 1 or 2 drops in the affected eye(s) as needed.
- Children under 6 years of age: ask a doctor
Inactive Ingredients
Benzalkonium chloride as preservative, boric acid, calcium chloride, hypromellose, magnesium chloride, potassium chloride, purified water, sodium borate, sodium chloride, zinc chloride
Eye Drops SY Other information
- Tamper Evident. Do not use this product if imprinted neckband on bottle is broken or missing.
- RETAIN THIS CARTON FOR FUTURE REFERENCE
- Store at room temperature
Principal Display Panel
EDSY
Eye Drops SYPolyethylene glycol 400, Propylene glycol SOLUTION/ DROPS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!