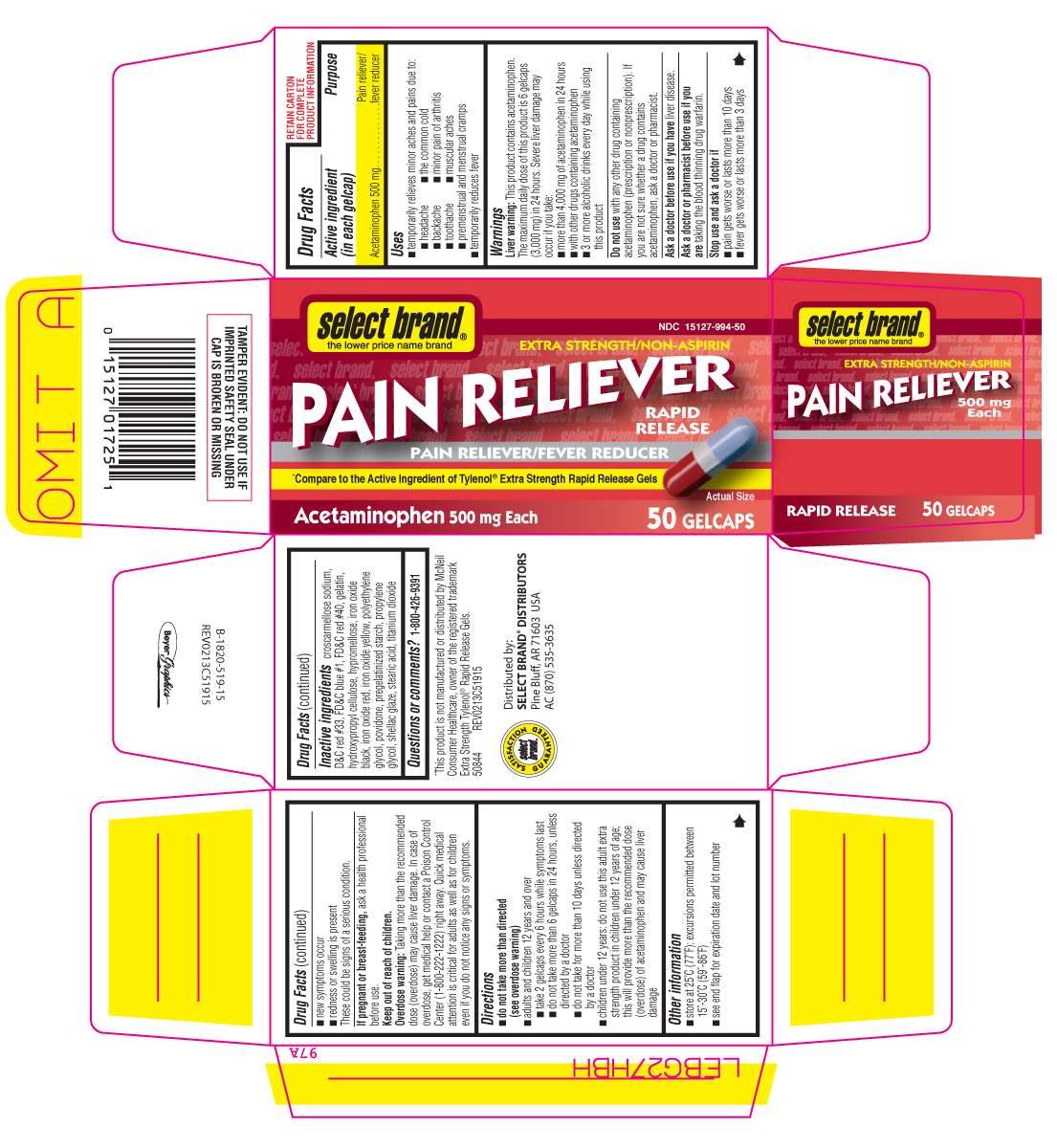
Extra Strength Pain Reliever
Stephen L. LaFrance Pharmacy, Inc.
Select Brand 44-519
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each gelcap)
- Purpose
- Extra Strength Pain Reliever Uses
- Warnings
- Directions
- Extra Strength Pain Reliever Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient (in each gelcap)
Acetaminophen 500 mg
Purpose
Pain reliever/fever reducer
Extra Strength Pain Reliever Uses
-
temporarily relieves minor aches and pains due to:
-
headache
-
the common cold
-
backache
-
minor pain of arthritis
-
toothache
-
muscular aches
-
premenstrual and menstrual cramps
-
-
temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. The maximum daily dose of this product is 6 gelcaps (3,000 mg) in 24 hours. Severe liver damage may occur if you take:
-
more than 4,000 mg of acetaminophen in 24 hours
-
with other drugs containing acetaminophen
-
3 or more alcoholic drinks every day while using this product
Do not use
with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Ask a doctor before use if you have
liver disease.
Ask a doctor or pharmacist before use if you are
taking the blood thinning drug warfarin.
Stop use and ask a doctor if
-
pain gets worse or lasts more than 10 days
-
fever gets worse or lasts more than 3 days
-
new symptoms occur
-
redness or swelling is present
These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
-
do not take more than directed (see overdose warning)
-
adults and children 12 years and over
-
take 2 gelcaps every 4 to 6 hours while symptoms last
-
do not take more than 8 gelcaps in 24 hours
-
do not take for more than 10 days unless directed by a doctor
-
-
children under 12 years: do not use this adult extra strength product in children under 12 years of age; this will provide more than the recommended dose (overdose) of acetaminophen and may cause liver damage
Extra Strength Pain Reliever Other information
-
store at 25ºC (77ºF); excursions permitted between 15°-30°C (59°-86°F)
-
see end flap for expiration date and lot number
Inactive ingredients
croscarmellose sodium, D&C red #33, FD&C blue #1, FD&C red #40, gelatin, hydroxypropyl cellulose, hypromellose, iron oxide black, iron oxide red, iron oxide yellow, polyethylene glycol, povidone, pregelatinized starch, propylene glycol, shellac glaze, simethicone, stearic acid, titanium dioxide
Questions or comments?
1-800-426-9391
Principal Display Panel
select brand®
the lower price name brand
NDC 15127-994-24
EXTRA STRENGTH/NON-ASPIRIN
PAIN RELIEVER
RAPID RELEASE
PAIN RELIEVER/FEVER REDUCER
*Compare to the Active Ingredient of Tylenol® Extra Strength Rapid Release Gels
Acetaminophen 500 mg Each
24 gelcaps
*This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Tylenol® Extra Strength Rapid Release Gels.
50844 REV0213C51915
Distributed by:
SELECT BRAND* DISTRIBUTORS
Pine Bluff, AR 71603 USA
AC (870) 535-3635
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Extra Strength Pain RelieverAcetaminophen CAPSULE, GELATIN COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||