ESIKA
Ventura Corporation (San Juan, P.R)
ēsika Perfect sun kids
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- ESIKA Uses
- Warnings
- Directions
- ESIKA Other information
- Inactive ingredients
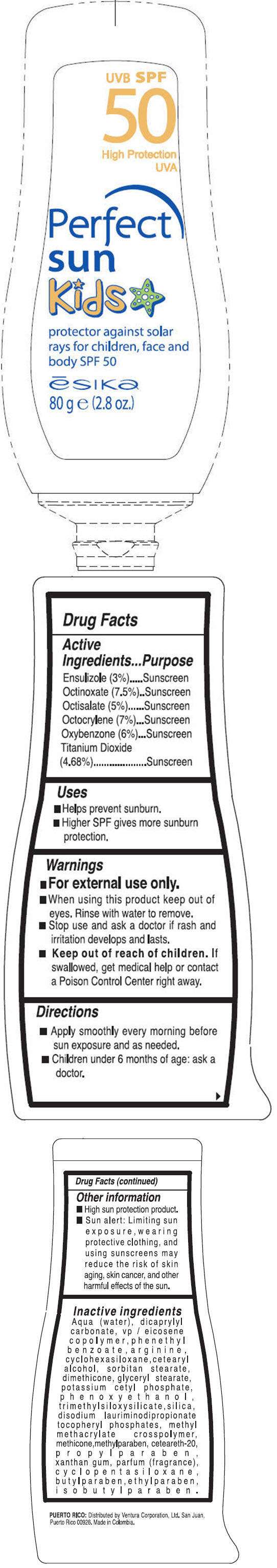
- PRINCIPAL DISPLAY PANEL - 82 g Bottle Label
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredients
Ensulizole (3%), Octinoxate (7.5%), Octisalate (5%), Octocrylene (7%), Oxybenzone (6%),Titanium Dioxide (4.68%)
Purpose
Sunscreen
ESIKA Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
Warnings
For external use only.
When using this product
- Keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
- rash and irritation develops and lasts.
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply smoothly every morning before sun exposure and as needed.
- Children under 6 months of age: ask a doctor.
ESIKA Other information
- High sun protection product.
- Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risk of skin aging, skin cancer, and other harmful effects of the sun.
Inactive ingredients
Aqua (water), dicaprylyl carbonate, vp/eicosene copolymer, phenethyl benzoate, arginine, cyclohexasiloxane, cetearyl alcohol, sorbitan stearate, dimethicone, glyceryl stearate, potassium cetyl phosphate, phenoxyethanol, trimethylsiloxysilicate, silica, disodium lauriminodipropionate tocopheryl phosphates, methyl methacrylate crosspolymer, methicone, methylparaben, ceteareth-20, propylparaben, xanthan gum, parfum (fragrance), cyclopentasiloxane, butylparaben, ethylparaben, isobutylparaben.
PRINCIPAL DISPLAY PANEL - 82 g Bottle Label
UVB SPF
50
High Protection
UVA
Perfect
sun
kids
protector against solar
rays for children, face and
body SPF 50
ēsika
80 g e (2.8 oz.)
ESIKAENSULIZOLE, OCTINOXATE, OCTISALATE, OCTOCRYLENE, OXYBENZONE, and TITANIUM DIOXIDE CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||