Esika
ēsika
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- Esika Uses
- Warnings
- Directions
- Esika Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL - 15 g Carton
FULL PRESCRIBING INFORMATION
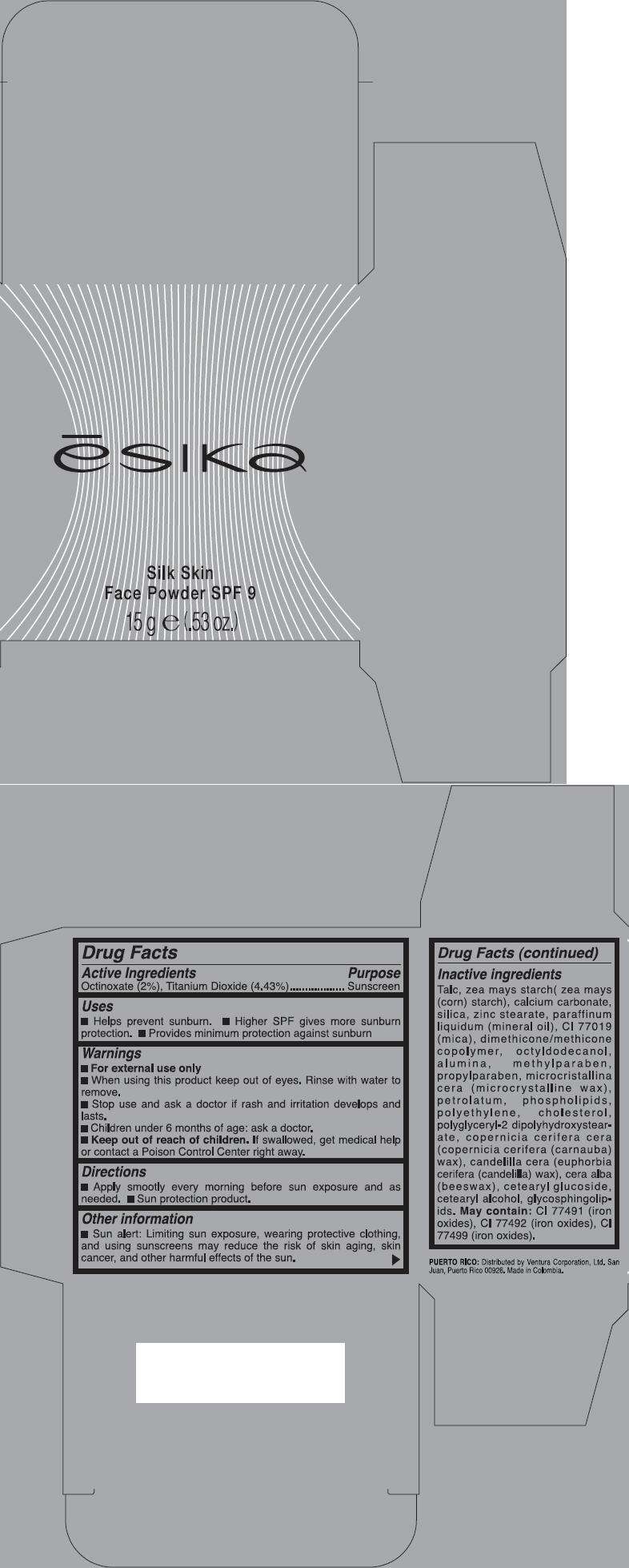
Drug Facts
Active Ingredients
Octinoxate (2%), Titanium Dioxide (4.43%)
Purpose
Sunscreen
Esika Uses
- Helps prevent sunburn.
- Higher SPF gives more sunburn protection.
- Provides minimum protection against sunburn
Warnings
- For external use only
- When using this product keep out of eyes. Rinse with water to remove.
- Stop use and ask a doctor if rash and irritation develops and lasts.
- Children under 6 months of age: ask a doctor.
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply smootly every morning before sun exposure and as needed.
- Sun protection product.
Esika Other information
- Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risk of skin aging, skin cancer, and other harmful effects of the sun.
Inactive ingredients
Talc, zea mays starch( zea mays (corn) starch), calcium carbonate, silica, zinc stearate, paraffinum liquidum (mineral oil), CI 77019 (mica), dimethicone/methicone copolymer, octyldodecanol, alumina, methylparaben, propylparaben, microcristallina cera (microcrystalline wax), petrolatum, phospholipids, polyethylene, cholesterol, polyglyceryl-2 dipolyhydroxystearate, copernicia cerifera cera (copernicia cerifera (carnauba) wax), candelilla cera (euphorbia cerifera (candelilla) wax), cera alba (beeswax), cetearyl glucoside, cetearyl alcohol, glycosphingolipids. May contain: CI 77491 (iron oxides), CI 77492 (iron oxides), CI 77499 (iron oxides).
PRINCIPAL DISPLAY PANEL - 15 g Carton
ēsika
Silk Skin
Face Powder SPF 9
15 g e (.53 oz.)
EsikaOCTINOXATE and TITANIUM DIOXIDE POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||