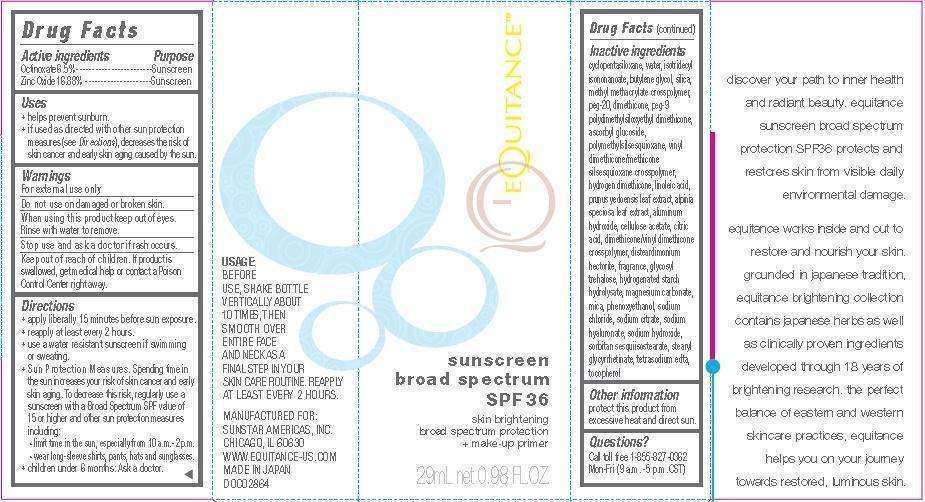
Equitance Sunscreen Broad Spectrum SPF 36
Equitance Sunscreen Broad Spectrum SPF 36 Octinoxate 6.5%Zinc Oxide 16.88%
FULL PRESCRIBING INFORMATION: CONTENTS*
- Drug Facts
- Active Ingredients
- Purpose
- Equitance Sunscreen Broad Spectrum SPF 36 Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- sunscreen broad spectrum SPF 36
- Questions?
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredients
Octinoxate 6.5%
Zinc Oxide 16.88%
Purpose
Sunscreen
Equitance Sunscreen Broad Spectrum SPF 36 Uses
- helps prevent sunburn.
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only.
Do not use
on damaged or broken skin.
When using this product
keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor
if rash occurs.
Keep out of reach of children.
If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before sun exposure.
- reapply at least every 2 hours.
- use a water resistant sunscreen if swimming or sweating.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m.-2 p.m.
• wear long-sleeve shirts, pants, hats and sunglasses. - children under 6 months: Ask a doctor.
Other Information
protect this product from excessive heat and direct sun.
Inactive Ingredients
cyclopentasiloxane, water, isotridecyl isononanoate, butylene glycol, silica, methyl methacrylate crosspolymer, peg-20, dimethicone, peg-9 polydimethylsiloxyethyl dimethicone, ascorbyl glucoside, polymethylsilsesquioxane, vinyl dimethicone/methicone silsesquioxane crosspolymer, hydrogen dimethicone, linoleic acid, prunus yedoensis leaf extract, alpinia speciosa leaf extract, aluminum hydroxide, cellulose acetate, citric
acid, dimethicone/vinyl dimethicone crosspolymer, disteardimonium hectorite, fragrance, glycosyl trehalose, hydrogenated starch hydrolysate, magnesium carbonate, mica, phenoxyethanol, sodium chloride, sodium citrate, sodium hyaluronate, sodium hydroxide, sorbitan sesquiisostearate, stearyl glycyrrhetinate, tetrasodium edta, tocopherol.
sunscreen broad spectrum SPF 36
Skin brightening broad spectrum protection + make-up primer
Discover your path to inner health and radiant beauty. Equitance sunscreen broad spectrum protection SPF36 protects and restores skin from visible daily environmental damage. Equitance works inside and out to restore and nourish your skin.
Grounded in japanese tradition, equitance brightening collection contains japanese herbs as well as clinically proven ingredients developed through 18 years of brightening research. The perfect balance of eastern and western skincare practices, equitance helps you on your journey
towards restored, luminous skin.
USAGE:
BEFORE USE, SHAKE BOTTLE VERTICALLY ABOUT 10 TIMES, THEN SMOOTH OVER ENTIRE FACE AND NECK AS A FINAL STEP IN YOUR SKIN CARE ROUTINE. REAPPLY AT LEAST EVERY 2 HOURS.
MANUFACTURED FOR:
SUNSTAR AMERICAS, INC.
CHICAGO, IL 60630
WWW.EQUITANCE-US.COM
MADE IN JAPAN
DOC02864
Questions?
Call toll free 1-855-827-0362
Mon-Fri ( 9 a.m. - 5 p.m. CST)
Equitance sunscreen broad spectrum protection SPF36
sunscreen
broad spectrum
SPF 36
skin brightening
broad spectrum protection
+ make-up primer
29mL net 0.98 FL. OZ
Equitance Sunscreen Broad Spectrum SPF 36Octinoxate Zinc Oxide LOTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||