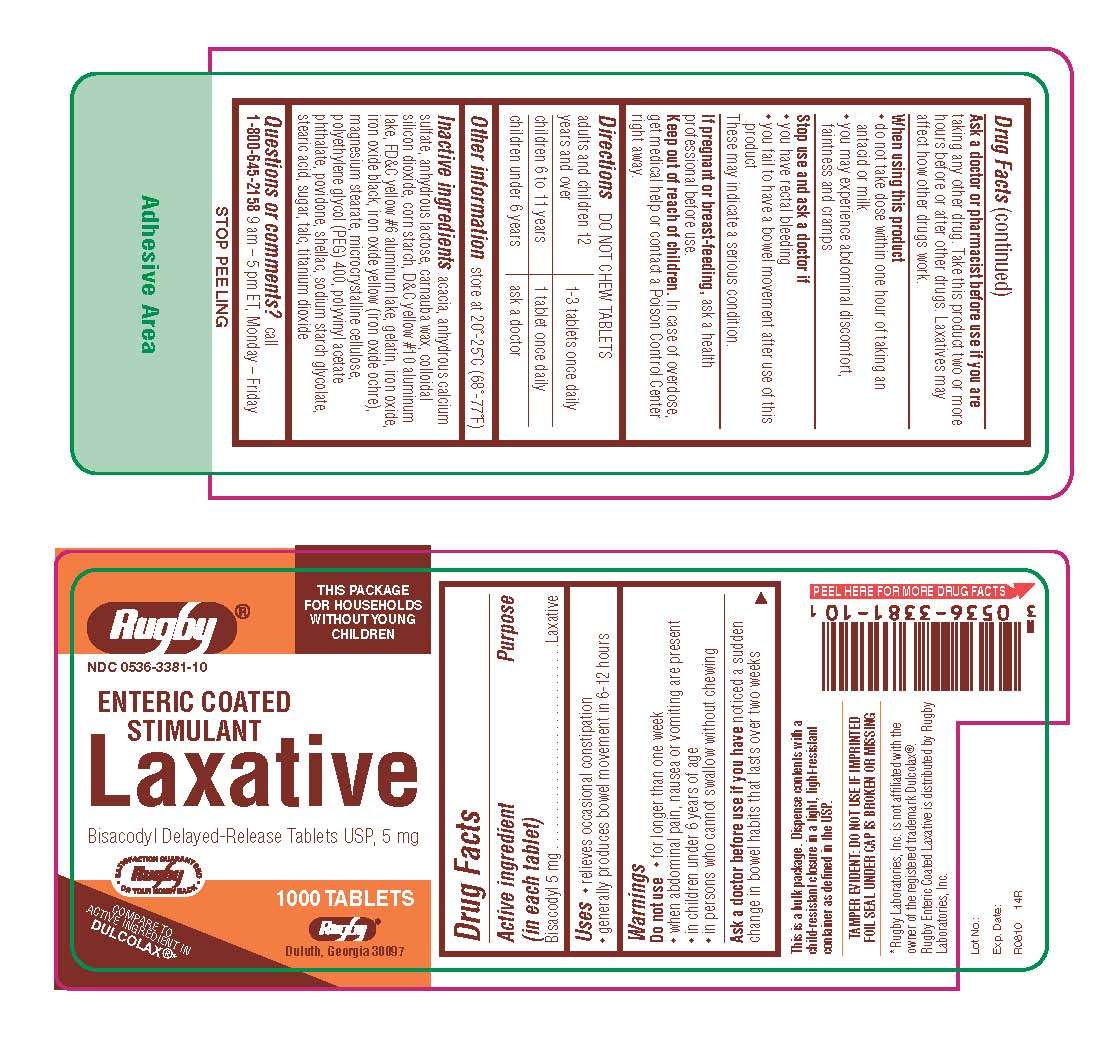
Enteric Coated Stimulant Laxative
Rugby Laboratories, Inc
Time Cap Labs, Inc
DRUG FACTS
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredient (in each tablet)Purpose
PurposeUses
Uses- relieves occasional constipation
- generally produces bowel movement in 6-12 hours
Do not use
- for longer than one week
- when abdominal pain, nausea or vomiting are present
- in children under 6 years of age
- in persons who cannot swallow without chewing
Ask a doctor or pharmacist before use if you are
When using this product
- do not take dose within one hour of taking an antacid or milk
- you may experience abdominal discomfort, faintness and cramps
- you have rectal bleeding
- you fail to have a bowel movement after use of this product
If pregnant or breast-feeding,
Keep out of reach of children.
Directions
Other information
Inactive ingredients
Questions or comments?
1-800-645-2158
Enteric Coated Stimulant LaxativeBisacodyl TABLET, DELAYED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!