EltaMD UV Facial
Swiss-American Products
Swiss-American Products
EltaMD UV Daily SPF 40
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients Purpose
Octinoxate 7.5%.............UVB Sunscreen
Zinc Oxide 7.0%.....UVA/UVB Sunscreen
Uses
EltaMD UV Facial SPF 30+ is a light, creamy, cosmetically elegant moisturizing sunscreen. It is specifically formulated to provide moisturizing UVA/UVB protection for the face, either alone or under makeup. For all skin types, especially moderate to dry skin types. EltaMD UV Facial is ideal after microdermabrasion, IPL treatments, chemical or glycolic peels and laser procedures.
EltaMD UV Facial SPF 30+ is formulated with a transparent micronized zinc oxide that provides broad spectrum protection from both the UVA (aging) and UVB (burning) rays. It is non-comedogenic, sensitivity-free and fragrance-free.
WarningsFor external use only
- Avoid direct contact with the eyes.
- Discontinue use if signs of irritation appear.
- Children under 6 months of age, consult your doctor.
Directions
- Apply daily to face, neck and backs of hands, 30 minutes before exposure to the sun.
Inactive Ingredients
Purified Water, Petrolatum, Isopropyl Palmitate, Octyl Stearate, Cetearyl Glucoside, Glyceryl Stearate, Dimethicone, PEG-100 Stearate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Polyisobutene, PEG-7 Trimethylolpropane Coconut Ether, Sodium Hyaluronate, Tocopheryl Acetate, Polyether-1, Citric Acid, Oleth-3 Phosphate, Phenoxyethanol, Butylene Glycol, Iodopropynyl Butylcarbamate, Triethoxycaprylylsilane
Enter section text here 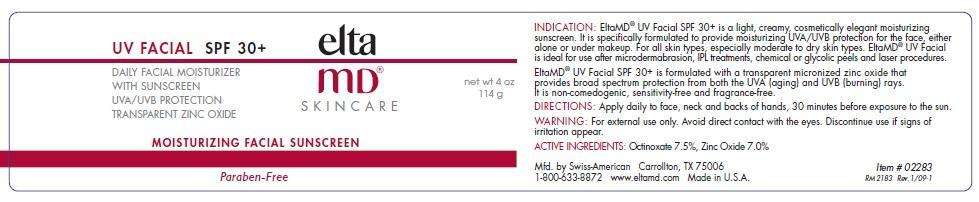
EltaMD UV FacialZinc Oxide, Octinoxate LOTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||