ELF Mineral Moisturizing Lip Tint SPF 8
Shanghai Justking Enterprise Co. Ltd.
Drug Fact
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient:
Titanium Dioxide: 4%
Purpose
Purpose:
Sunscreen
Uses
Uses:
Apply to lip area to prevent Sunburn
Apply on lips to hydrate, repair and relieve chapped lips.
Warning:
For external use only
When Using This Product:
Avoid contact with eyes. If contact occurs, rinse thoroughly with water
Discontinue use if signs of rash or irritation appear. If irritation or rash persists, consult a doctor.
Keep Out of Reach of Children:
If swallowed get medical help or contact a Poison Control Center immediately
Directions:
Twist up tube and apply to lip area.
Apply prior to sun exposure
Use as a touch-up tinted lip moisturizer throughout the day as often as needed.
Other Imformation:
Store at room temperature 15-30C (59-86F)
Inactive Ingredient:
Paraffinium Liquidum (Mineral Oil), Caprylic/Capric Triglyceride, Iso-Octyl Palmitate, Synthetic Wax, Microcrystalline Wax, Petrolatum, Bis-Diglyceryl Polyacyladipate-2, Butyrospermum Parkli (Shea Butter), Tocopheryl Acetate, Cocos Nucifere (Coconut) Oil, Simmondsia Chinensis (Jojoba) Seed Oil.
May contain:
Iron Oxides (CI 77491, CI 77492, CI 77499), Manganese Violet (CI 77742), Mica (CI 77019)
label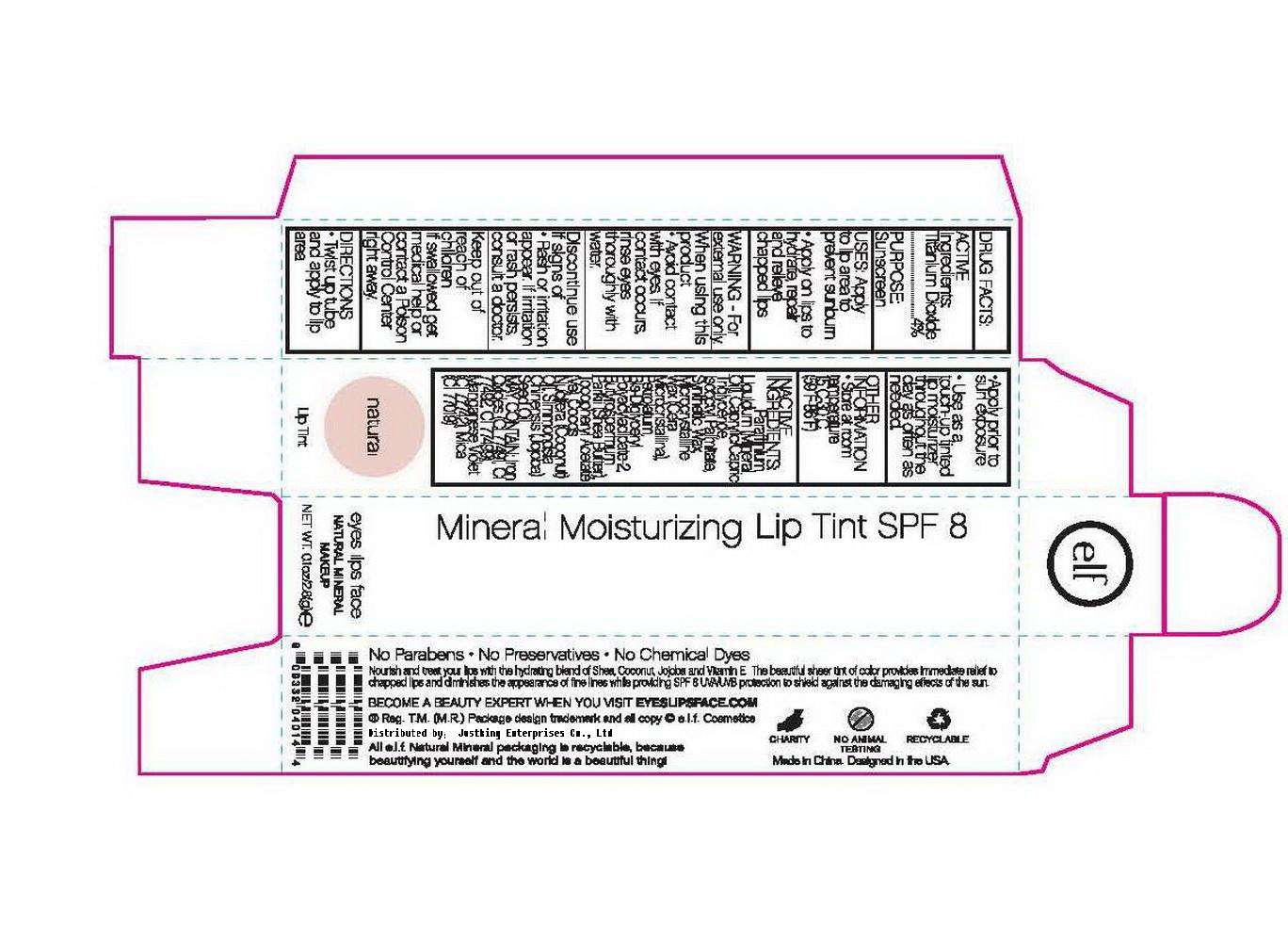
ELF Mineral Moisturizing Lip Tint SPF 8TITANIUM DIOXIDE POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||