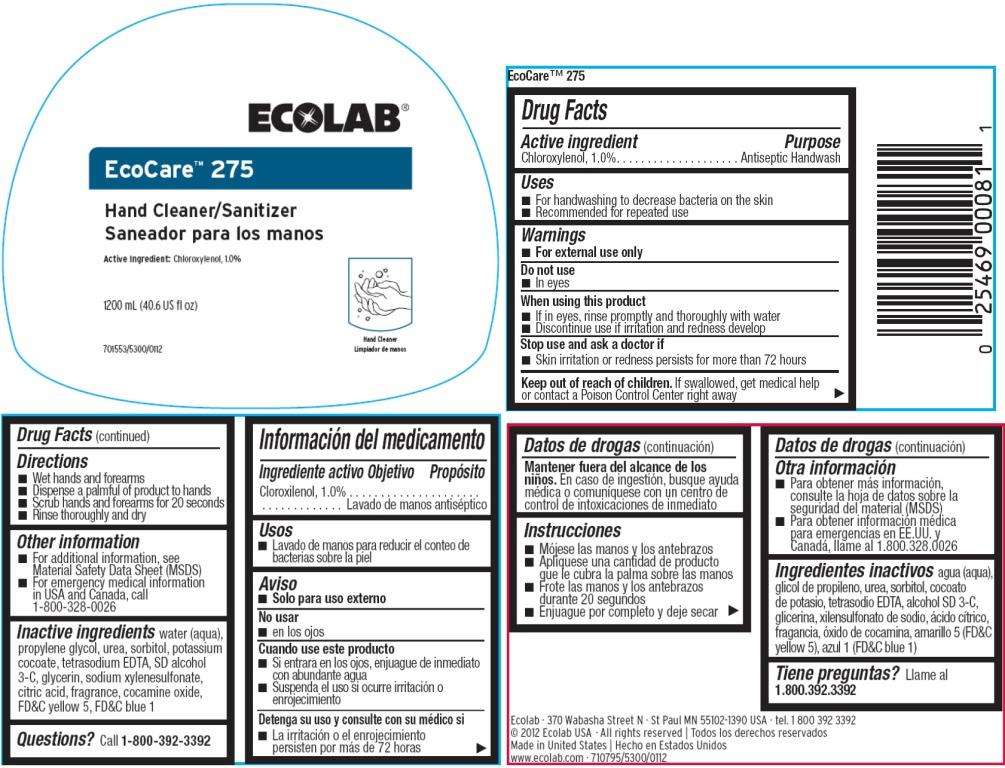
EcoCare 275
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- EcoCare 275 Uses
- Warnings
- Directions
- EcoCare 275 Other information
- Inactive ingredients
- Questions?
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active Ingredient
Chloroxylenol, 1.0%
Purpose
Antiseptic handwash
EcoCare 275 Uses
- For handwashing to decrease bacteria on the skin
- Recommended for repeated use
Warnings
-
For external use only
Do not use
- In eyes
When using this product
- If in eyes, rinse promptly and thoroughly with water
- Discontinue use if irritation and redness develop
Stop use and ask a doctor if
- Skin irritation or redness persists for more than 72 hours.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Wet hands and forearms
- Dispense a palmful of product to hands
- Scrub hands and forearms for 20 seconds
- Rinse throughly and dry
EcoCare 275 Other information
- For additional information, see Material Safety Data Sheet (MSDS)
- For emergency medical information in USA and Canada, call 1-800-328-0026
Inactive ingredients
water (aqua), propylene glycol, urea, sorbitol, potassium cocoate, tetrasodium EDTA, SD alcohol 3-C, glycerin, sodium xylenesulfonate, citric acid, fragrance, cocamine oxide, FDC yellow 5, FDC blue 1
Questions?
Call 1-800-392-3392
Principal Display Panel
ECOLAB
EcoCare 275
Hand Cleaner/Sanitizer
Active Ingredient: Chloroxylenol, 1.0%
EcoCare 275Chloroxylenol SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!