Ear Wax Treatment
Rugby Earwax Treatment Drops
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Ear Wax Treatment Other information
- Inactive ingredients
- Questions or comments?
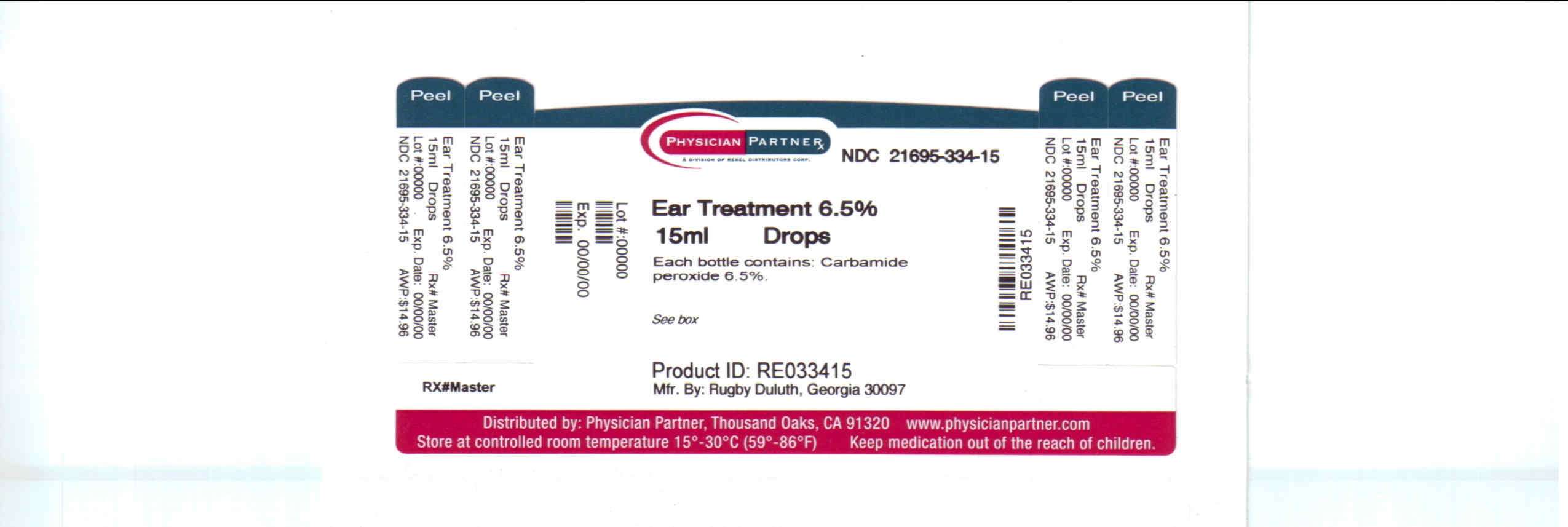
- PRINCIPAL DISPLAY PANEL - 15 mL Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Carbamide peroxide 6.5%
Purpose
Earwax removal aid
Use
softens, loosens and removes excessive earwax
Warnings
Do not use for more than 4 days
Ask a doctor before use if you have
- ear drainage or discharge
- ear pain, irritation, rash or are dizzy
- an injury or perforation (hole) of the eardrum or if you had ear surgery
When using this product avoid contact with the eyes
Stop use and ask a doctor if earwax remains longer than 4 days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
FOR USE IN THE EAR ONLY
adults and children over 12 years:
- tilt head sideways and place 5 to 10 drops into ear
- tip of applicator should not enter ear canal
- keep drops in ear for several minutes by tilting head or placing cotton in ear
- use twice daily for up to 4 days
- remove any wax remaining after treatment by gently flushing ear with warm water using a soft rubber bulb ear syringe
children under 12 years: ask a doctor
Ear Wax Treatment Other information
- store at room temperature 15° - 30°C (59° - 89°F)
- keep in a dry place
Inactive ingredients
citric acid (anhydrous), glycerin, propylene glycol, purified water, sodium acid pyrophosphate, sodium citrate (anhydrous), trolamine
Questions or comments?
Call 1-800-645-2158
9am - 5pm ET
Monday - Friday
Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
PRINCIPAL DISPLAY PANEL - 15 mL Carton
COMPARE TO
ACTIVE INGREDIENT IN
DEBROX®*
Rugby®
NDC 21695-334-15
Earwax
Treatment
Drops
Carbamide Peroxide 6.5 %
Helps Remove
Excessive Earwax
1/2 fl oz (15 mL)
SATISFACTION GUARANTEED
Rugby
OR YOUR MONEY BACK
Ear Wax TreatmentCarbamide Peroxide SOLUTION/ DROPS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||