Duane Reade
Duane Reade
Creations Garden Natural Products Inc.
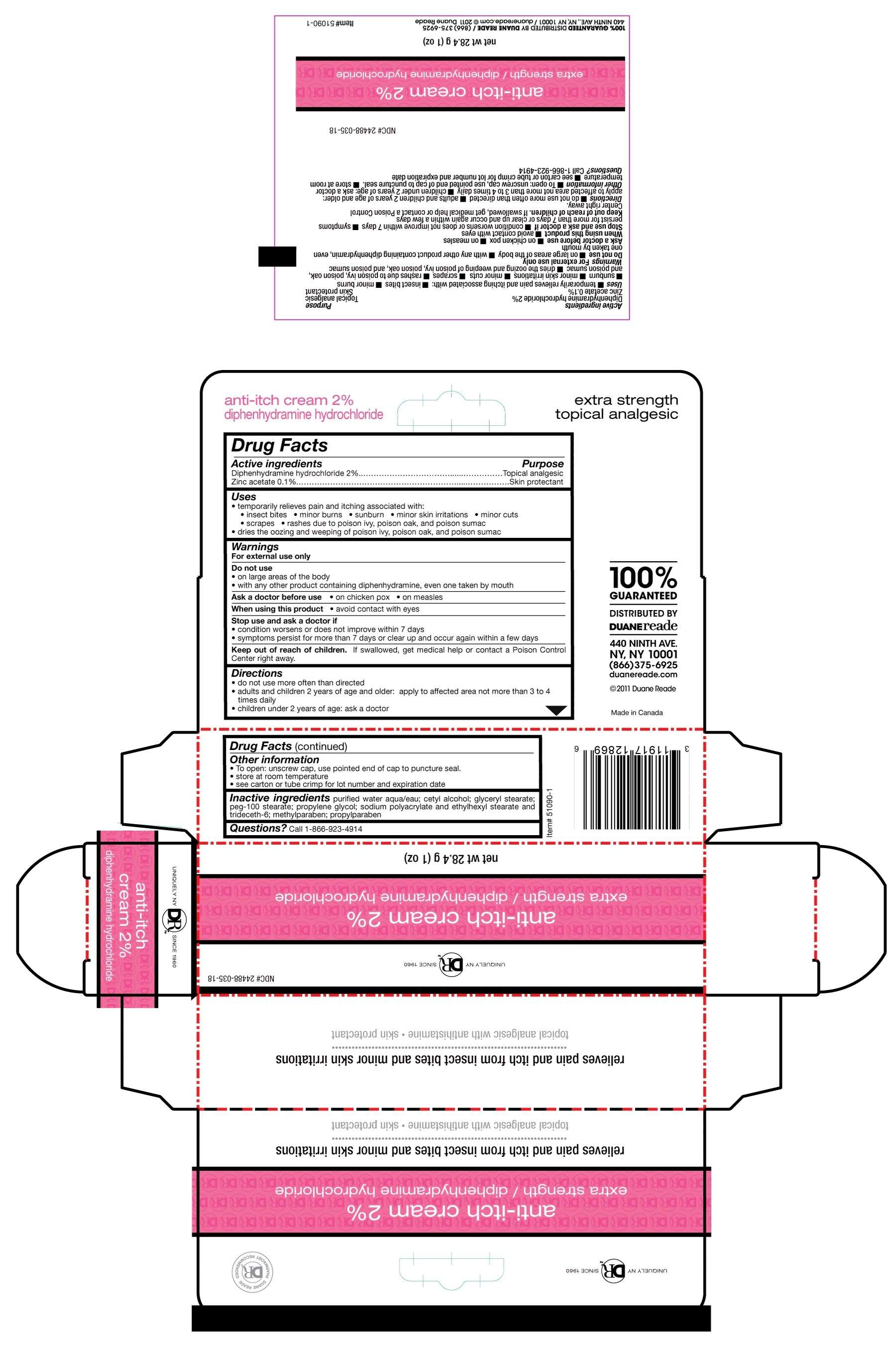
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Duane Reade Uses
- Warnings
- Do not use
- Ask a doctor before use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
- Duane Reade Other information
- Inactive ingredients
- Questions
FULL PRESCRIBING INFORMATION
Active Ingredient
Diphenhydramine hydrochloride 2%........Topical analgesic
Zinc acetate 0.1%.......Skin protectant
Duane Reade Uses
- temporarily relieves pain and itching associated with:
- insect bites
- minor burns
- sunburn
- minor skin irritations
- minor cuts
- scrapes
- rashes due to poison ivy, poison oak, and poison sumac
- dries the oozing and weeping of poison ivy, poison oak, and poison sumac
Warnings
For external use only
Do not use
- on large areas of the body
- with any other product containing diphenhydramine, even one taken by mouth
Ask a doctor before use
- on chicken pox
- on measles
When using this product
- avoid contact with eyes
Stop use and ask a doctor if
- condition worsens or does not improve within 7 days
- symptoms persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- do not use more often than directed
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
Duane Reade Other information
- To open: unscrew cap, use pointed end of cap to puncture seal.
- store at room temperature
- see carton or tube crimp for lot number and expiration date
Inactive ingredients
purified water aqua/eau; cetyl alcohol; glyceryl stearate; peg-100 stearate; propylene glycol; sodium polyacrylate and ethylhexyl stearate and trideceth-6; methylparaben; propylparaben
Questions
Call 1-866-923-4914
placeholder text
Duane ReadeDIPHENHYDRAMINE HYDROCHLORIDE CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!