Dr.Jart V7 Beauty Balm
Have and Be Co., Ltd.
Have and Be Co., Ltd.
Human OTC Drug Label
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Warnings Section
- Ask Doctor Section
- Keep out of Reach of Children
- Purpose
- Instructions for Use
- Inactive Ingredients
- Package Label
FULL PRESCRIBING INFORMATION
Active Ingredients
Titanium Dioxide 10.44% Zinc Oxide 1.44%
Warnings Section
For External Use Only
Ask Doctor Section
Stop use and ask a doctor if rash or irritation develops and lasts.
Keep out of Reach of Children
if swallowed get medical help or contact a poison control center right away.
Purpose
Sunscreen
Instructions for Use
-Apply Generously and as needed.
- Children under 6 months of age: Ask a doctor
- Reapply as needed or after towel drying, swimming, and sweating.
Inactive Ingredients
Water
CYCLOMETHICONE 5
BUTYLENE GLYCOL
DICAPRYLATE/DICAPRATE
Dimethicone
Cyclohexasiloxane
POLYMETHYLSILSESQUIOXANE
(11 MICRONS)
Glycerin
Phenyl
Trimethicone
PEG-10 DIMETHICONE (600 CST)
Magnesium Sulfate
DISTEARYLDIMONIUM
FERRIC OXIDE YELLOW
Talc
Calcium
Stearate
CAPRYLYL GLYCOL
Propylene Carbonate
Ethylhexylglycerin
CETYL PEG/PPG-10/1
DIMETHICONE (HLB 5)
Polyglyceryl-4 Isostearate
Phenoxyethanol
Hexyl Laurate
Triethoxycaprylylsilane
Sodium
Ascorbyl Phosphate
Niacinamide
Butylene
Glycol
Panthenol
Hydrogen
Dimethicone
.ALPHA.-TOCOPHEROL ACETATE
1,2-Hexanediol
EDETATE DISODIUM
Alcohol
RUMEX CRISPUS ROOT
MYRCIARIA DUBIA FRUIT
HIPPOPHAE RHAMNOIDES FRUIT
PANTOLACTONE, (R)-
HYALURONATE SODIUM
Package Label
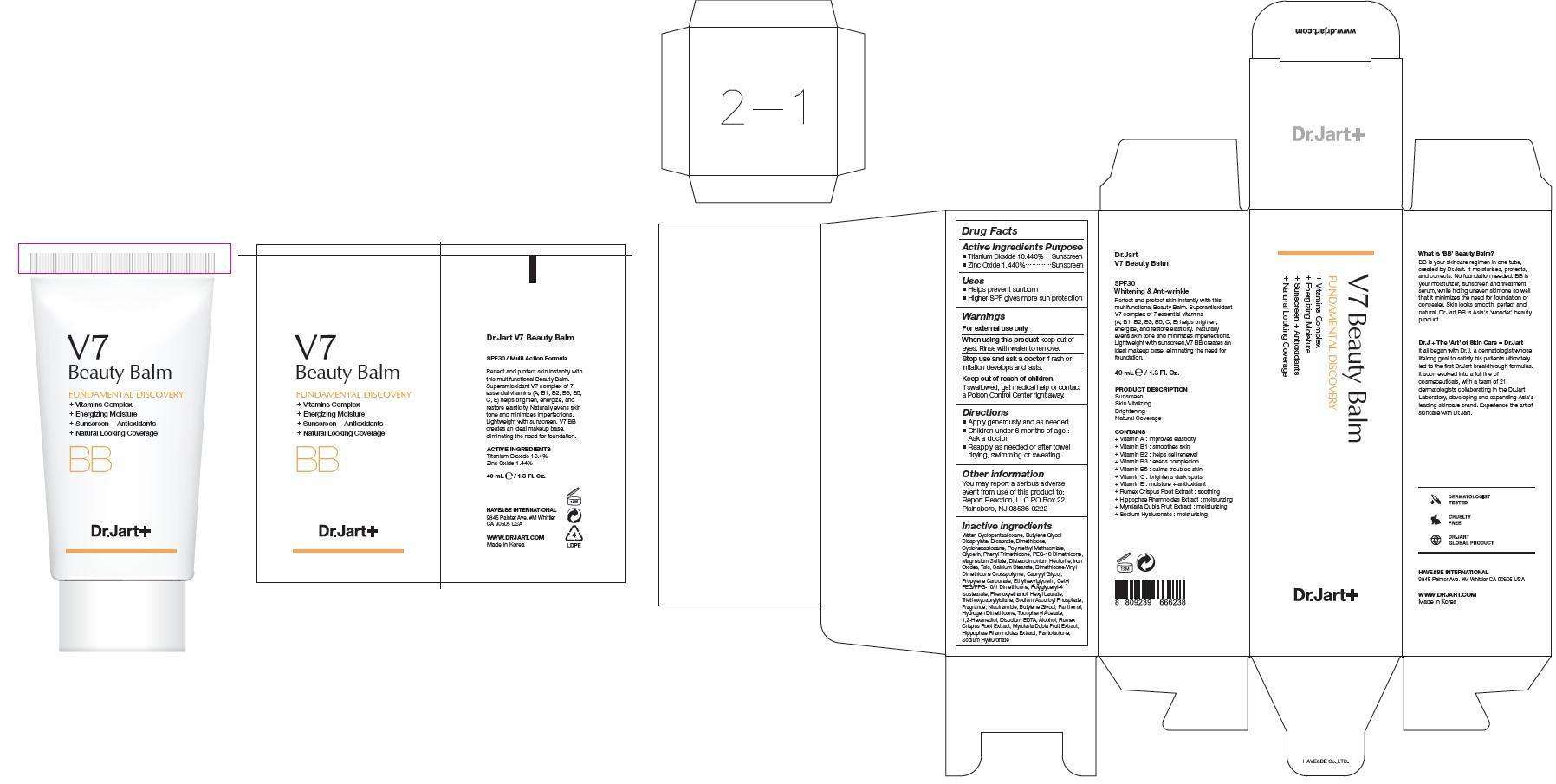
Dr.Jart V7 Beauty BalmTitanium Dioxide, Zinc Oxide CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||