Drainage
Drainage
FULL PRESCRIBING INFORMATION
Active ingredient
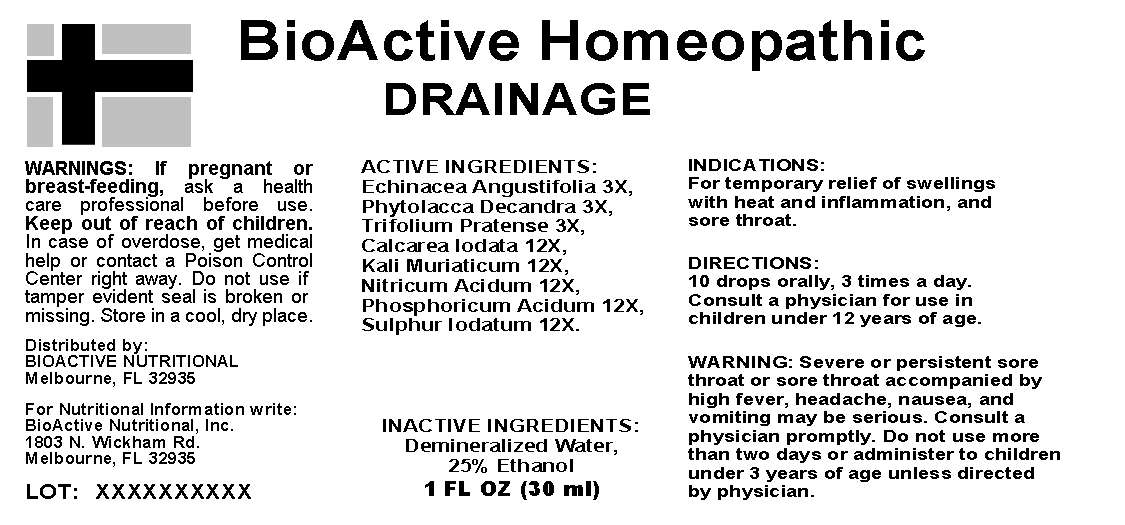
Active Ingredients: Echinacea Angustifolia 3X, Phytolacca Decandra 3X, Trifolium Pratense 3X, Calcarea Iodata 12X, Kali Muriaticum 12X, Nitricum Acidum 12X, Phosphoricum Acidum 12X, Sulphur Iodatum 12X.
Purpose
Indications: For temporary relief of swellings with heat and inflammation, and sore throat.
WARNINGS: If pregnant or breast-feeding, ask a health care profession before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing.
Store in a cool, dry place.
Directions: 10 drops orally, 3 times a day. Consult a physician for use in children under 12 years of age.
Warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea and vomiting may be serious. Consult a physician promptly. Do not use more that two days or administer to children under 3 years of age unless directed by physician.
Inactive Ingredients: Demineralized Water, 25% Ethanol
Distributed By:
BioActive Nutritional
Melbourne, FL 32935
For Nutritional Information Write:
BioActive Nutritional, Inc.
1803 N. Wickham Rd.
Melbourne, FL 32935
 BioActive Homeopathic
BioActive Homeopathic
DRAINAGE
1 FL OZ (30 ML)
DrainageDrainage LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||