DR. RECKEWEG R59 Vesiculine
PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO
DR. RECKEWEG R59 Vesiculine
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredients
Calcarea carbonica 12X, Fucus vesiculosus 4X, Graphites 12X, Natrum sulphuricum 4X, Spongia tosta 4X, 1 g each in 10 g.
Purpose
Purpose
Relieves symptoms associated with weight gain due to glandular dysfunction
Uses
Uses
For the temporary relief of minor symptoms associated with:
- Weight gain due to glandular dysfunction
Warnings
Do not use
- if you are hypersensitive to iodine
- if you have a thyroid condition (e.g. hypothyroidism or hyperthyroidism)
- if you are pregnant or breastfeeding
- in cases with intestinal affections
Stop use and ask a doctor if symptoms persist, get worse, or if new symptoms occur.
Keep out of reach of children.
Directions
Adults and children ≥ 12 years 5-10 drops 1-3 times daily in a little water or undiluted or as recommended.
Other information
- Tamper resistant: do not use if safety ring at base of cap is broken.
- Retain outer carton for full product instructions.
Inactive ingredients
Ethanol, purified water.
Questions?
Call 1-800-361-7872 or email questions@reckeweg.com
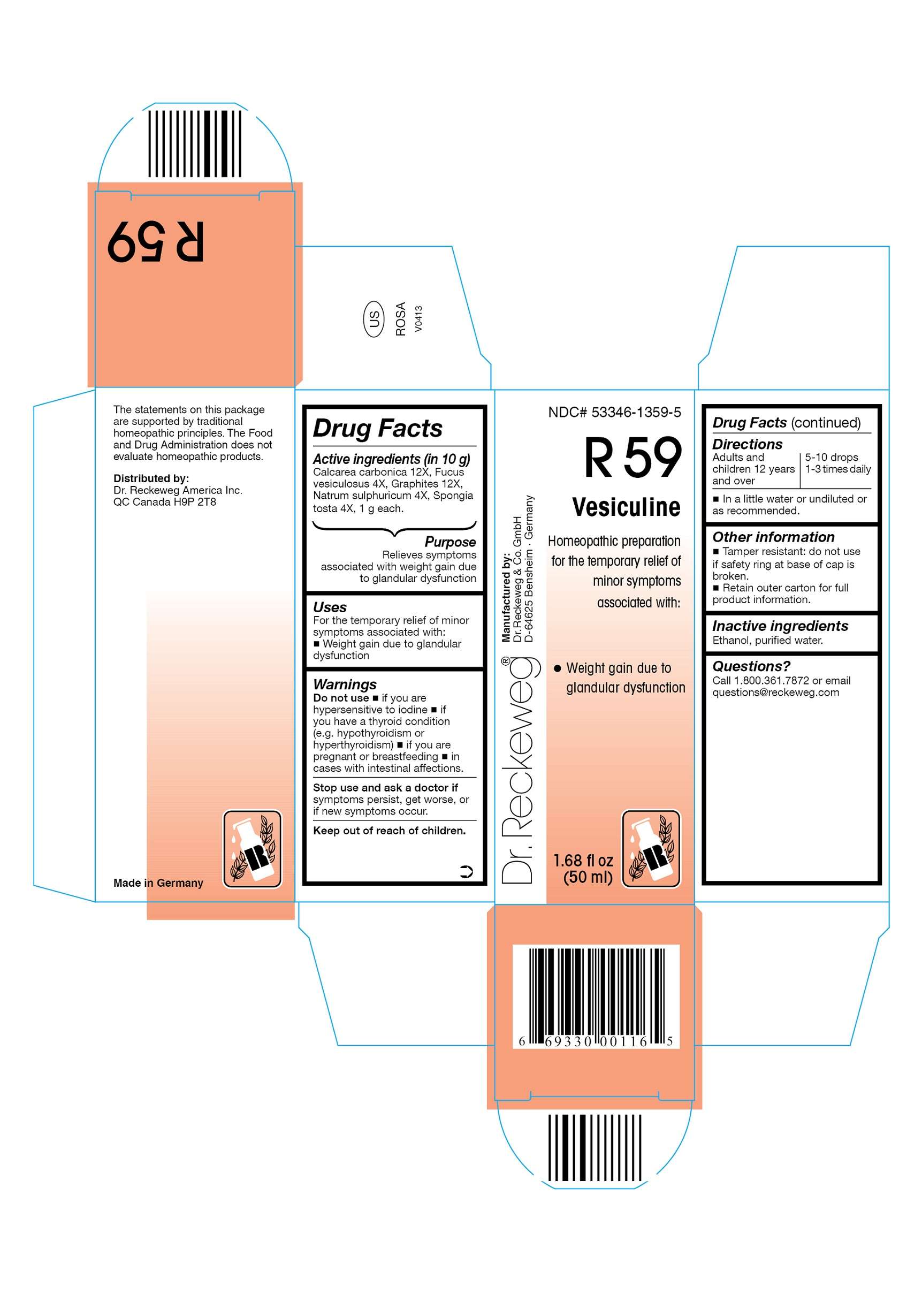
NDC# 53346-1359-5
Dr. Reckeweg R59 Vesiculine
Homeopathic preparation for the temporary relief of minor symptoms associated with:
- Weight gain due to glandular dysfunction
Manufactured by:
Dr. Reckeweg Co. GmbH
D-64625 Bensheim
Germany
1.68 fl oz
(50 ml)
DR. RECKEWEG R59 VesiculineCalcarea carbonica 12X, Fucus vesiculosus 4X, Graphites 12X, Natrum sulphuricum 4X, Spongia tosta 4X LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||