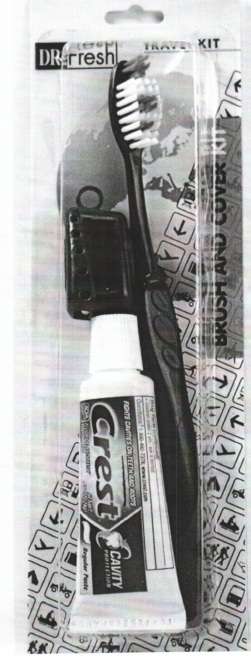
Dr. Fresh Brush and Cover
Dr. Fresh Inc. Brush and Cover Kit
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Dr. Fresh Brush and Cover Uses
- Warnings
- Directions
- Inactive Ingredients
- Comments? Questions?
- Brush and Cover Kit
FULL PRESCRIBING INFORMATION
Active Ingredient
Sodium fluoride 0.243% (0.15% w/v fluoride ion)
Purpose
Anticavity toothpaste
Dr. Fresh Brush and Cover Uses
Helps protect against cavities
Warnings
If you accidentally swallow more than used for brushing, seek professional assistance or contact a poison control center immediately.
Keep out of the reach of children under 6 years of age.
Directions
Adults and children 6 years and older: Brush teeth thoroughly after meals or at least twice a day or use as directed by a dentist or physician.
Children under 6 years: To minimize swallowing use a pea-sized amount and supervise brushing until good habits are established.
Children under 2 years: Ask a dentist or physician.
Do not swallow.
Inactive Ingredients
Sorbitol, water, hydrated silica, trisodium phosphate, sodium lauryl sulfate, flavor, sodium phosphate, xanthan gum, carbomer 956, sodium saccharin, titanium dioxide, blue no. 1
Comments? Questions?
Dr. Fresh Inc
Buena Park, CA 90620 USA
Toll Free: 1-866-DR-FRESH
Visit us at: www.drfresh.com
Brush and Cover Kit
Dr. Fresh Travel Kit
The Dr. Fresh Travelers brand is expressly made for the consumer who seeks high quality at a reasonable proce. Using innovation in design and intelligence in the manufacturing process, we pride ourselves on products that offer the utmost in value.
Have a great trip!
Dr. Fresh


Dr. Fresh Brush and CoverSODIUM FLUORIDE PASTE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||