Doxorubicin Hydrochloride
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use doxorubicin hydrochloride safely and effectively. See full prescribing information for doxorubicin hydrochloride liposome injection. Doxorubicin Hydrochloride Liposome Injection for intravenous infusionInitial U.S. Approval: 1995 RECENT MAJOR CHANGESContraindications, Nursing Mother (4) Removed 9/2012BOXED WARNINGWARNING: INFUSION REACTIONS, MYELOSUPPRESSION, CARDIOTOXICITY, LIVER IMPAIRMENT, SUBSTITUTION See full prescribing information for complete boxed warning. Myocardial damage may lead to congestive heart failure and may occur as the total cumulative dose of doxorubicin hydrochloride approaches 550 mg/m2. Cardiac toxicity may also occur at lower cumulative doses with mediastinal irradiation or concurrent cardiotoxic agents (5.1). Acute infusion-related reactions, sometimes reversible upon terminating or slowing infusion, occurred in up to 10% of patients. Serious and sometimes fatal allergic/anaphylactoid-like infusion reactions have been reported. Medications/emergency equipment to treat such reactions should be available for immediate use (5.2). Severe myelosuppression may occur (5.3) Reduce dosage in patients with impaired hepatic function (2.6). Accidental substitution of doxorubicin hydrochloride liposome injection resulted in severe side effects. Do not substitute on mg per mg basis with doxorubicin hydrochloride (2.1). INDICATIONS AND USAGEDoxorubicin hydrochloride is an anthracycline topoisomerase inhibitor indicated for: • Ovarian cancer (1.1) After failure of platinum-based chemotherapy. • AIDS-related Kaposi’s Sarcoma (1.2) After failure of prior systemic chemotherapy or intolerance to such therapy.DOSAGE AND ADMINISTRATIONAdminister doxorubicin hydrochloride liposome injection at an initial rate of 1 mg/min to minimize the risk of infusion reactions. If no infusion related reactions occur, increase rate of infusion to complete administration over 1 hour. Do not administer as bolus injection or undiluted solution (2.1). Ovarian cancer: 50 mg/m2 IV every 4 weeks for 4 courses minimum (2.2) AIDS-related Kaposi’s Sarcoma: 20 mg/m2 IV every 3 weeks (2.3) DOSAGE FORMS AND STRENGTHS3CONTRAINDICATIONS Hypersensitivity reactions to a conventional formulation of doxorubicin hydrochloride or the components of doxorubicin hydrochloride liposome injection (4, 5.2) WARNINGS AND PRECAUTIONS Hand-Foot Syndrome may occur. Dose modification or discontinuation may be required (5.4) Radiation recall reaction may occur (5.5) Side Effects6To report SUSPECTED ADVERSE REACTIONS contact CARACO Pharmaceutical Laboratories Ltd. at 1-800-818-4555 orFDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS Doxorubicin hydrochloride may interact with drugs known to interact with conventional formulations of Doxorubicin hydrochloride. (7) USE IN SPECIFIC POPULATIONS Doxorubicin hydrochloride liposome injection can cause fetal harm when used during pregnancy. (5.6, 8.1) Discontinue nursing during treatment with doxorubicin hydrochloride liposome injection (8.3).
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: INFUSION REACTIONS, MYELOSUPPRESSION, CARDIOTOXICITY, LIVER IMPAIRMENT, ACCIDENTAL SUBSTITUTION
- 1 DOXORUBICIN HYDROCHLORIDE INDICATIONS AND USAGE
- 2 DOXORUBICIN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 DOXORUBICIN HYDROCHLORIDE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 DOXORUBICIN HYDROCHLORIDE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 DOXORUBICIN HYDROCHLORIDE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NON-CLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 10 ML
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON - 10 ML
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 25 ML
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON - 25 ML
FULL PRESCRIBING INFORMATION
WARNING: INFUSION REACTIONS, MYELOSUPPRESSION, CARDIOTOXICITY, LIVER IMPAIRMENT, ACCIDENTAL SUBSTITUTION
- The use of doxorubicin hydrochloride liposome injection may lead to cardiac toxicity. Myocardial damage may lead to congestive heart failure and may occur as the total cumulative dose of doxorubicin hydrochloride approaches 550 mg/m2. In a clinical study in patients with advanced breast cancer, 250 patients received doxorubicin hydrochloride liposome injection at a starting dose of 50 mg/m2 every 4 weeks. At all cumulative anthracycline doses between 450 to 500 mg/m2 or between 500 to 550 mg/m2, the risk of cardiac toxicity for patients treated with doxorubicin hydrochloride liposome injection was 11%. Prior use of other anthracyclines or anthracenediones should be included in calculations of total cumulative dosage. Cardiac toxicity may also occur at lower cumulative doses in patients with prior mediastinal irradiation or who are receiving concurrent cyclophosphamide therapy [see Warnings and Precautions (5.1)].
- Acute infusion-related reactions including, but not limited to, flushing, shortness of breath, facial swelling, headache, chills, back pain, tightness in the chest or throat, and/or hypotension have occurred in up to 10% of patients treated with doxorubicin hydrochloride liposome injection. In most patients, these reactions resolve over the course of several hours to a day once the infusion is terminated. In some patients, the reaction has resolved with slowing of the infusion rate. Serious and sometimes life-threatening or fatal allergic/anaphylactoid- like infusion reactions have been reported. Medications to treat such reactions, as well as emergency equipment, should be available for immediate use. Doxorubicin hydrochloride liposome injection should be administered at an initial rate of 1 mg/min to minimize the risk of infusion reactions [see Warnings and Precautions (5.2)].
- Severe myelosuppression may occur [see Warnings and Precautions (5.3)].
- Dosage should be reduced in patients with impaired hepatic function [see Dosage and Administration (2.6) and Use in Specific Populations (8.6)].
- Accidental substitution of doxorubicin hydrochloride liposome injection for doxorubicin hydrochloride has resulted in severe side effects. Doxorubicin hydrochloride liposome injection should not be substituted for doxorubicin hydrochloride on a mg per mg basis [see Dosage and Administration (2.1)].
1 INDICATIONS AND USAGE
1.1 Ovarian Cancer
1.2 AIDS-Related Kaposi’s Sarcoma
2 DOSAGE AND ADMINISTRATION
2.1 Usage and Administration Precautions
[see Warnings and Precautions (5.2)]
2.2 Patients With Ovarian Cancer
2[see Warnings and Precautions (5.1)][see Dosage and Administration (2.5)]
2.3 Patients With AIDS-Related Kaposi’s Sarcoma
2
2.5 Dose Modification Guidelines
2[see Clinical Pharmacology (12.3)]
| Toxicity Grade | Dose Adjustment |
|---|---|
|
1
(mild erythema, swelling, or desquamation not interfering with daily activities) |
Redose unless patient has experienced previous Grade 3 or 4 HFS. If so, delay up to 2 weeks and decrease dose by 25%. Return to original dose interval. |
|
2
(erythema, desquamation, or swelling interfering with, but not precluding normal physical activities; small blisters or ulcerations less than 2 cm in diameter) |
Delay dosing up to 2 weeks or until resolved to Grade 0-1. If after 2 weeks there is no resolution, doxorubicin hydrochloride liposome injection should be discontinued. If resolved to Grade 0-1 within 2 weeks, and there are no prior Grade 3-4 HFS, continue treatment at previous dose and return to original dose interval. If patient experienced previous Grade 3-4 toxicity, continue treatment with a 25% dose reduction and return to original dose interval. |
|
3
(blistering, ulceration, or swelling interfering with walking or normal daily activities; cannot wear regular clothing) |
Delay dosing up to 2 weeks or until resolved to Grade 0-1. Decrease dose by 25% and return to original dose interval. If after 2 weeks there is no resolution, doxorubicin hydrochloride liposome injection should be discontinued. |
|
4
(diffuse or local process causing infectious complications, or a bed ridden state or hospitalization) |
Delay dosing up to 2 weeks or until resolved to Grade 0-1. Decrease dose by 25% and return to original dose interval. If after 2 weeks there is no resolution, doxorubicin hydrochloride liposome injection should be discontinued |
| Grade | ANC | Platelets | Modification |
|---|---|---|---|
|
1
|
1,500 to 1,900 |
75,000 to 150,000 |
Resume treatment with no dose reduction |
|
2
|
1,000 to <1,500 |
50,000 to <75,000 |
Wait until ANC ≥ 1,500 and platelets ≥ 75,000; redose with no dose reduction |
|
3
|
500 to 999 |
25,000 to <50,000 |
Wait until ANC ≥ 1,500 and platelets ≥ 75,000; redose with no dose reduction |
|
4
|
<500 |
<25,000 |
Wait until ANC ≥ 1,500 and platelets ≥ 75,000; redose at 25% dose reduction or continue full dose with cytokine support |
| Toxicity Grade | Dose Adjustment |
|---|---|
|
1
(painless ulcers, erythema, or mild soreness) |
Redose unless patient has experienced previous Grade 3 or 4 toxicity. If so, delay up to 2 weeks and decrease dose by 25%. Return to original dose interval. |
|
2
(painful erythema, edema, or ulcers, but can eat) |
Delay dosing up to 2 weeks or until resolved to Grade 0-1. If after 2 weeks there is no resolution, doxorubicin hydrochloride liposome injection should be discontinued. If resolved to Grade 0-1 within 2 weeks and there was no prior Grade 3-4 stomatitis, continue treatment at previous dose and return to original dose interval. If patient experienced previous Grade 3-4 toxicity, continue treatment with a 25% dose reduction and return to original dose interval. |
|
3
(painful erythema, edema, or ulcers, and cannot eat) |
Delay dosing up to 2 weeks or until resolved to Grade 0-1. Decrease dose by 25% and return to original dose interval. If after 2 weeks there is no resolution, doxorubicin hydrochloride liposome injection should be discontinued. |
|
4
(requires parenteral or enteral support) |
Delay dosing up to 2 weeks or until resolved to Grade 0-1. Decrease dose by 25% and return to doxorubicin hydrochloride liposome injection original dose interval. If after 2 weeks there is no resolution, doxorubicin hydrochloride liposome injection should be discontinued. |
2.6 Patients With Impaired Hepatic Function
2.7 Preparation for Intravenous Administration
Do not use with in-line filters.
2.8 Procedure for Proper Handling and Disposal
Doxorubicin hydrochloride liposome injection must not be given by the intramuscular or subcutaneous route.
[see References (15)]
3 DOSAGE FORMS AND STRENGTHS
- Single use vial: 20 mg/10 mL
- Single use vial: 50 mg/25 mL
4 CONTRAINDICATIONS
Doxorubicin hydrochloride [see Warnings and Precautions (5.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Cardiac Toxicity
22
2 22
| Doxorubicin Hydrochloride Liposome Injection (n=250) | |
|---|---|
| Patients who Developed Cardiotoxicity (LVEF Defined) |
10 |
| Cardiotoxicity (With Signs & Symptoms of CHF) |
0 |
| Cardiotoxicity (no Signs & Symptoms of CHF) |
10 |
| Patients With Signs and Symptoms of CHF Only |
2 |
5.2 Infusion Reactions
[see Dosage and Administration (2)]
5.3 Myelosuppression
33[see Adverse Reactions (6.2)]
[see Dosage and Administrations (2.5)]
2 [see Adverse Reactions (6.2)]
5.4 Hand-Foot Syndrome (HFS)
2 [see definitions of HFS grades in Dosage and Administration (2.5)]
2
[see Dosage and Administration (2.5)]
5.5 Radiation Recall Reaction
5.6 Fetal Mortality
Pregnancy Category D
[see Use in Specific Populations (8.1)]
5.7 Toxicity Potentiation
5.8 Monitoring: Laboratory Tests
[see Warnings and Precautions (5.3)]
6 ADVERSE REACTIONS
6.1 Overall Side Effects Profile
The following adverse reactions are discussed in more detail in other sections of the labeling.
- Cardiac Toxicity [see Warnings and Precautions (5.1)]
- Infusion reactions [see Warnings and Precautions (5.2)]
- Myelosuppression [see Warnings and Precautions (5.3)]
- Hand-Foot syndrome [see Warnings and Precautions (5.4)]
[see Adverse Reactions in Clinical Trials (6.2)]
6.2 Side Effects in Clinical Trials
2
Table 6
| Doxorubicin Hydrochloride Liposome Injection Patients (n = 239) |
Topotecan Patients (n = 235) |
|
|---|---|---|
| Neutropenia |
||
| 500 to <1000/mm3
|
19 (7.9%) |
33 (14%) |
| <500/mm3
|
10 (4.2%) |
146 (62.1%) |
| Anemia |
||
| 6.5 to <8 g/dL |
13 (5.4%) |
59 (25.1%) |
| < 6.5 g/dL |
1 (0.4%) |
10 (4.3%) |
| Thrombocytopenia |
||
| 10,000 to <50,000/mm3
|
3 (1.3%) |
40 (17%) |
| <10,000/mm3
|
0 (0%) |
40 (17%) |
| Non-Hematologic Adverse Reaction 10% or Greater | Doxorubicin Hydrochloride Liposome Injection (%) treated (n = 239) |
Topotecan (%) treated (n =235) |
||
|---|---|---|---|---|
|
All grades
|
Grades 3-4
|
All grades
|
Grades 3-4
|
|
|
Body as a Whole
|
||||
| Asthenia |
40.2 |
7.1 |
51.5 |
8.1 |
| Fever |
21.3 |
0.8 |
30.6 |
5.5 |
| Mucous Membrane Disorder |
14.2 |
3.8 |
3.4 |
0 |
| Back Pain |
11.7 |
1.7 |
10.2 |
0.9 |
| Infection |
11.7 |
2.1 |
6.4 |
0.9 |
| Headache |
10.5 |
0.8 |
14.9 |
0 |
|
Digestive
|
||||
| Nausea |
46 |
5.4 |
63 |
8.1 |
| Stomatitis |
41.4 |
8.3 |
15.3 |
0.4 |
| Vomiting |
32.6 |
7.9 |
43.8 |
9.8 |
| Diarrhea |
20.9 |
2.5 |
34.9 |
4.2 |
| Anorexia |
20.1 |
2.5 |
21.7 |
1.3 |
| Dyspepsia |
12.1 |
0.8 |
14 |
0 |
|
Nervous
|
||||
| Dizziness |
4.2 |
0 |
10.2 |
0 |
|
Respiratory
|
||||
| Pharyngitis |
15.9 |
0 |
17.9 |
0.4 |
| Dyspnea |
15.1 |
4.1 |
23.4 |
4.3 |
| Cough increased |
9.6 |
0 |
11.5 |
0 |
|
Skin and Appendages
|
||||
| Hand-foot syndrome |
50.6 |
23.8 |
0.9 |
0 |
| Rash |
28.5 |
4.2 |
12.3 |
0.4 |
| Alopecia |
19.2 |
N/A |
52.3 |
N/A |
Cardiovascular:
Digestive:
Hemic and Lymphatic:
Metabolic and Nutritional:
Nervous:
Respiratory:
Skin and Appendages:
Special Senses:
Urinary:
2 2 22
333
| Patients With Refractory or Intolerant AIDS-Related Kaposi's Sarcoma (n = 74) |
Total Patients With AIDS-Related Kaposi's Sarcoma (n = 720) |
|||
|---|---|---|---|---|
| Neutropenia |
||||
| < 1000/mm3
|
34 |
(45.9%) |
352 |
(48.9%) |
| < 500/mm3
|
8 |
(10.8%) |
96 |
(13.3%) |
| Anemia |
||||
| < 10 g/dL |
43 |
(58.1%) |
399 |
(55.4%) |
| < 8 g/dL |
12 |
(16.2%) |
131 |
(18.2%) |
| Thrombocytopenia |
||||
| < 150,000/mm3
|
45 |
(60.8%) |
439 |
(60.9%) |
| < 25,000/mm3
|
1 |
(1.4%) |
30 |
(4.2%) |
| Adverse Reactions | Patients With Refractory or Intolerant AIDS-Related Kaposi’s Sarcoma (n = 77) |
Total Patients With AIDS-Related Kaposi’s Sarcoma (n = 705) |
||
|---|---|---|---|---|
| Nausea |
14 |
(18.2%) |
119 |
(16.9%) |
| Asthenia |
5 |
(6.5%) |
70 |
(9.9%) |
| Fever |
6 |
(7.8%) |
64 |
(9.1%) |
| Alopecia |
7 |
(9.1%) |
63 |
(8.9%) |
| Alkaline Phosphatase Increase |
1 |
(1.3%) |
55 |
(7.8%) |
| Vomiting |
6 |
(7.8%) |
55 |
(7.8%) |
| Diarrhea |
4 |
(5.2%) |
55 |
(7.8%) |
| Stomatitis |
4 |
(5.2%) |
48 |
(6.8%) |
| Oral Moniliasis |
1 |
(1.3%) |
39 |
(5.5%) |
Body as a Whole: Cardiovascular:
Cutaneous:
Digestive:
Metabolic and Nutritional:
Other:
Body As A Whole:
Cardiovascular:
Digestive:
Metabolic and Nutritional Disorders:
Respiratory:
Skin and Appendages:
Special Senses:
6.3 Post Marketing Experience
Musculoskeletal and Connective Tissue Disorders:
Respiratory, Thoracic and Mediastinal Disorders:
Hematologic disorders
Skin and subcutaneous tissue disorders
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
[see Warnings and Precautions (5.6)]
2 2
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
[see Dosage and Administration (2.6)]
[see Dosage and Administration (2.6)]
10 OVERDOSAGE
11 DESCRIPTION
Streptomyces peucetius . caesius
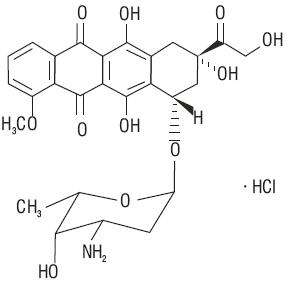
272911
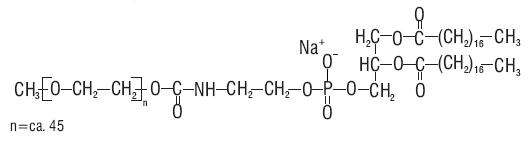
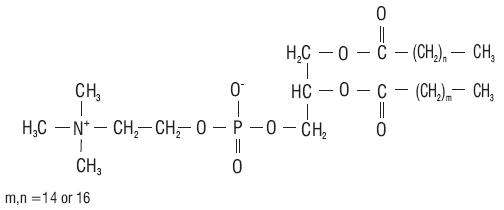
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
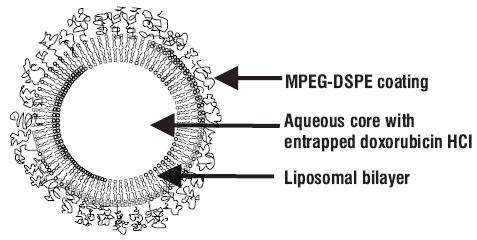
12.3 Pharmacokinetics
2 2
| Dose | ||
|---|---|---|
| Parameter (units) | 10 mg/m2 | 20 mg/m2 |
| N = 23 Mean ± Standard Error |
||
| Peak Plasma Concentration (mcg/mL) |
4.12 ± 0.215 |
8.34 ± 0.49 |
| Plasma Clearance (L/h/m2) |
0.056 ± 0.01 |
0.041 ± 0.004 |
| Steady State Volume of Distribution (L/m2) |
2.83 ± 0.145 |
2.72 ± 0.12 |
| AUC (mcg/mL∙h) |
277 ± 32.9 |
590 ± 58.7 |
| First Phase (λ1) Half-Life (h) |
4.7 ± 1.1 |
5.2 ± 1.4 |
| Second Phase (λ1) Half-Life (h) |
52.3 ± 5.6 |
55 ± 4.8 |
2 2 2
2
2
2 22
2
13 NON-CLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
in vitroin vivo
2 2 2 2 2 2
14 CLINICAL STUDIES
14.1 Ovarian Cancer
2
Table 12
| Study 1 (U.S.) (n = 27) |
Study 2 (U.S.) (n = 82) |
Study 3 (non-U.S.) (n = 36) |
|
|---|---|---|---|
| Age at Diagnosis (Years) |
|||
| Median |
64 |
61.5 |
51.5 |
| Range |
46 to 75 |
34 to 85 |
22 to 80 |
| Drug-Free Interval (Months) |
|||
| Median |
1.8 |
1.7 |
2.6 |
| Range |
0.5 to 15.6 |
0.6 to 7 |
0.7 to 15.2 |
| Sum of Lesions at Baseline (cm2) |
|||
| Median |
25 |
18.3 |
32.4 |
| Range |
1.2 to 230 |
1.3 to 285 |
0.3 to 114 |
| FIGO Staging |
|||
| I |
1 (3.7%) |
3 (3.7%) |
4 (11.1%) |
| II |
3 (11.1%) |
3 (3.7%) |
1 (2.8%) |
| III |
15 (55.6%) |
60 (73.2%) |
24 (66.7%) |
| IV |
8 (29.6%) |
16 (19.5%) |
6 (16.7%) |
| Not Specified |
— |
— |
1 (2.8%) |
| CA-125 at Baseline |
|||
| Median |
123.5 |
199 |
1004.5 |
| Range |
20 to 14,012 |
7 to 46,594 |
20 to 12,089 |
| Number of Prior Chemotherapy Regimens |
|||
| 1 |
7 (25.9%) |
13 (15.9%) |
9 (25%) |
| 2 |
11 (40.7%) |
44 (53.7%) |
19 (52.8%) |
| 3 |
6 (22.2%) |
25 (30.5%) |
8 (22.8%) |
| 4 |
3 (11.1%) |
— |
— |
Table 13
| Study 1 (U.S.) | Study 2 (U.S.) | Study 3 (non-U.S.) | |
|---|---|---|---|
| Response Rate |
22.2% (6/27) |
17.1% (14/82) |
0% (0/36) |
| 95% Confidence Interval |
8.6% to 42.3% |
9.7% to 27% |
0% to 9.7% |
2 2
Table 14
| Doxorubicin Hydrochloride Liposome Injection (n = 239) |
Topotecan (n = 235) |
|
|---|---|---|
| Age at Diagnosis (Years) |
||
| Median |
60 |
60 |
| Range |
27 to 87 |
25 to 85 |
| Drug-Free Interval (Months) |
||
| Median |
7 |
6.7 |
| Range |
0.9 to 82.1 |
0.5 to 109.6 |
| FIGO Staging |
||
| I |
11 (4.6%) |
15 (6.4%) |
| II |
13 (5.4%) |
8 (3.4%) |
| III |
175 (73.2%) |
164 (69.8%) |
| IV |
40 (16.7%) |
48 (20.4%) |
| Platinum Sensitivity |
||
| Sensitive |
109 (45.6%) |
110 (46.8%) |
| Refractory |
130 (54.4%) |
125 (53.2%) |
| Bulky Disease |
||
| Present |
108 (45.2%) |
105 (44.7%) |
| Absent |
131 (54.8%) |
130 (55.3%) |
| Protocol Defined ITT Population | ||
|---|---|---|
| Doxorubicin Hydrochloride Liposome Injection | Topotecan | |
| |
(n = 239) |
(n = 235) |
|
TTP (Protocol Specified Primary Endpoint) |
||
Median (Months) |
4.1 |
4.2 |
p-value |
0.617 |
|
Hazard Ratio |
0.955 |
|
| 95% CI for Hazard Ratio |
(0.762, 1.196) |
|
|
Overall Survival
|
||
Median (Months) |
14.4 |
13.7 |
p-value |
0.05 |
|
Hazard Ratio |
0.822 |
|
| 95% CI for Hazard Ratio |
(0.676, 1) |
|
|
Response Rate
|
||
| Overall Response n (%) |
47 (19.7) |
40 (17) |
| Complete Response n (%) |
9 (3.8) |
11 (4.7) |
| Partial Response n (%) |
38 (15.9) |
29 (12.3) |
Median Duration of Response (Months) |
6.9 |
5.9 |
14.2 AIDS-Related Kaposi’s Sarcoma
2
3
2 2
| Investigator Assessment | All Evaluable Patients (n = 34) |
Evaluable Patients Who Received Prior Doxorubicin (n = 20) |
|---|---|---|
Response |
||
| Partial (PR) |
27% |
30% |
| Stable |
29% |
40% |
| Progression |
44% |
30% |
| Duration of PR (Days) |
||
| Median |
73 |
89 |
| Range |
42+ to 210+ |
42+ to 210+ |
| Time to PR (Days) |
||
| Median |
43 |
53 |
| Range |
15 to 133 |
15 to 109 |
| Indicator Lesion Assessment |
All Evaluable Patients (n = 42) |
Evaluable Patients Who Received Prior Doxorubicin (n = 23) |
Response |
||
| Partial (PR) |
48% |
52% |
| Stable |
26% |
30% |
| Progression |
26% |
17% |
| Duration of PR (Days) |
||
| Median |
71 |
79 |
| Range |
22+ to 210+ |
35 to 210+ |
| Time to PR (Days) |
||
| Median |
22 |
48 |
| Range |
15 to 109 |
15 to 109 |
15 REFERENCES
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- NIH [2002]. 1999 recommendations for the safe handling of cytotoxic drugs. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, NIH Publication No. 92-2621.
- American Society of Health-System Pharmacists. (2006) ASHP Guidelines on Handling Hazardous Drugs.
- Polovich, M., White, J. M., & Kelleher, L.O. (eds.) 2005. Chemotherapy and biotherapy guidelines and recommendations for practice (2nd. ed.) Pittsburgh, PA: Oncology Nursing Society.
16 HOW SUPPLIED/STORAGE AND HANDLING
| mg in vial | fill volume | vial size | NDC #s |
|---|---|---|---|
| 20 mg vial |
10 mL |
10 mL |
47335-049-40 |
| 50 mg vial |
25 mL |
30 mL |
47335-050-40 |
17 PATIENT COUNSELING INFORMATION
Hand-Foot Syndrome
Stomatitis:
Fever and Neutropenia:
Nausea, vomiting, tiredness, weakness, rash, or mild hair loss:
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Ind. Ltd.
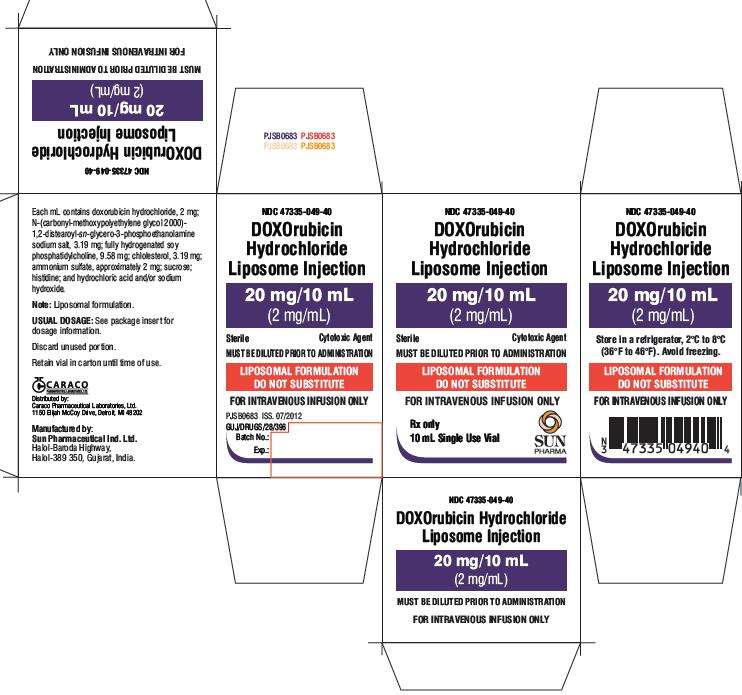
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 10 ML
NDC 47335-049-40
DOXOrubicin Hydrochloride Liposome Injection
20 mg/10 mL (2 mg/mL)
Sterile
Must be diluted
Cytotoxic Agent
LIPOSOMAL FORMULATION
DO NOT SUBSTITUTE
FOR INTRAVENOUS INFUSION ONLY

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON - 10 ML
NDC 47335-049-40
DOXOrubicin Hydrochloride Liposome Injection
20 mg/10 mL (2 mg/mL)
Sterile
Cytotoxic Agent
MUST BE DILUTED PRIOR TO ADMINISTRATION
LIPOSOMAL FORMULATION
DO NOT SUBSTITUTE
FOR INTRAVENOUS INFUSION ONLY
Rx only
10 mL Single Use Vial
SUN PHARMA
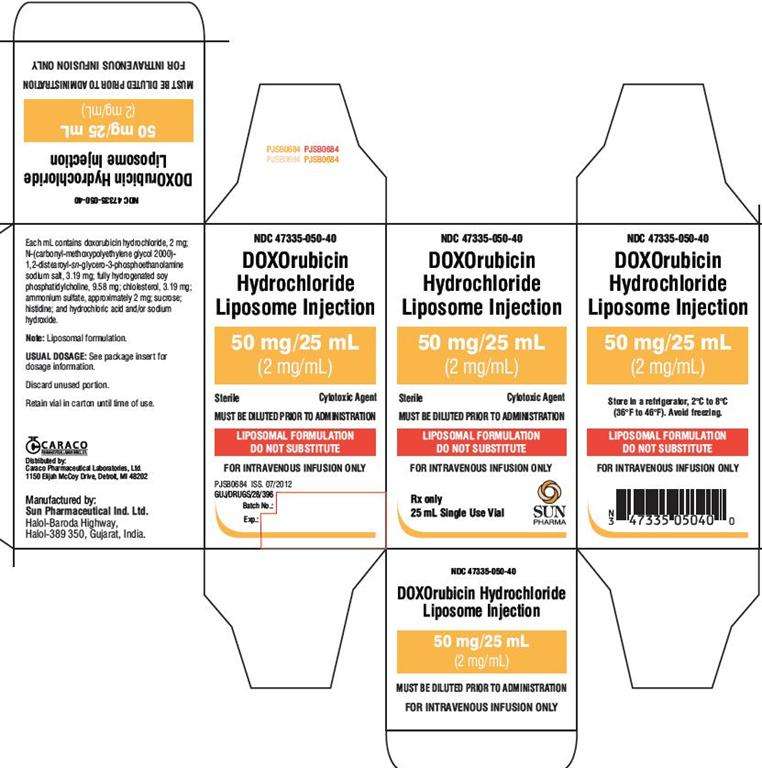
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 25 ML
NDC 47335-050-40
DOXOrubicin Hydrochloride Liposome Injection
50 mg/25 mL (2 mg/mL)
Sterile
Must be diluted
Cytotoxic Agent
LIPOSOMAL FORMULATION
DO NOT SUBSTITUTE
FOR INTRAVENOUS INFUSION ONLY

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON - 25 ML
NDC 47335-050-40
DOXOrubicin Hydrochloride Liposome Injection
50 mg/25 mL (2 mg/mL)
Sterile
Cytotoxic Agent
MUST BE DILUTED PRIOR TO ADMINISTRATION
LIPOSOMAL FORMULATION
DO NOT SUBSTITUTE
FOR INTRAVENOUS INFUSION ONLY
Rx only
25 mL Single Use Vial
SUN PHARMA
Doxorubicin HydrochlorideDoxorubicin Hydrochloride INJECTABLE, LIPOSOMAL
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Doxorubicin HydrochlorideDoxorubicin Hydrochloride INJECTABLE, LIPOSOMAL
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!