Doxazosin Mesylate
FULL PRESCRIBING INFORMATION: CONTENTS*
- DOXAZOSIN MESYLATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- DOXAZOSIN MESYLATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG & OR LABORATORY TEST INTERACTIONS
- DRUG INTERACTIONS
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- DOXAZOSIN MESYLATE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL PATIENT PACKAGE INSERT
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
DOXAZOSIN MESYLATE DESCRIPTION
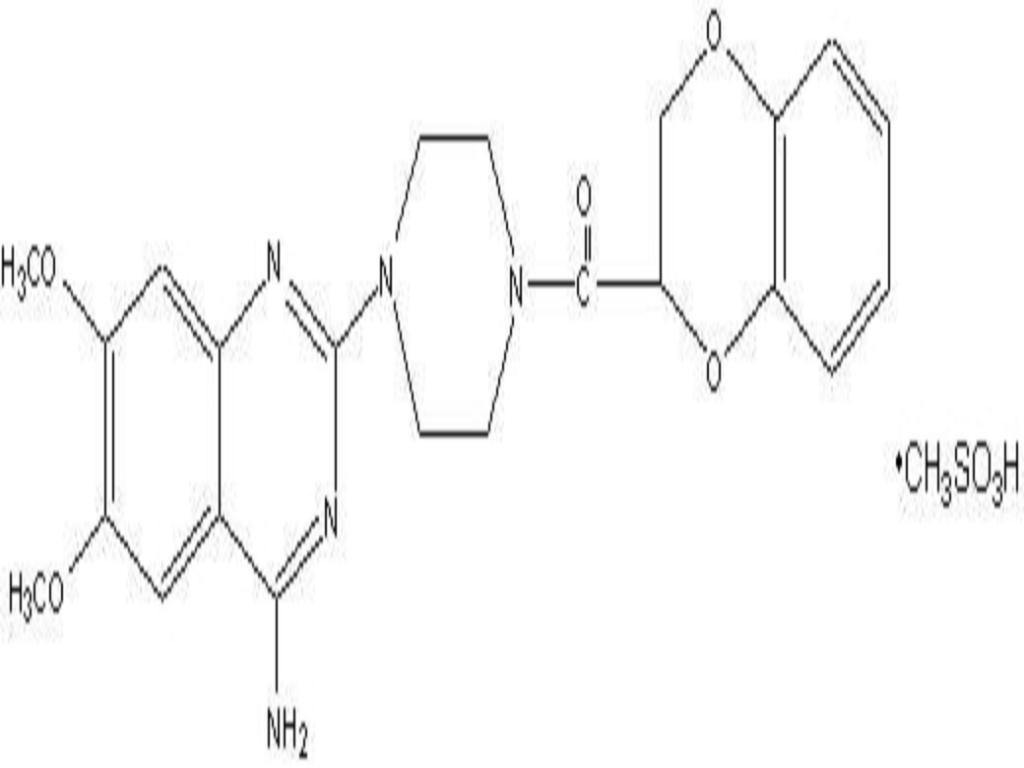
CLINICAL PHARMACOLOGY
PharmacodynamicsBenign Prostatic Hyperplasia (BPH)
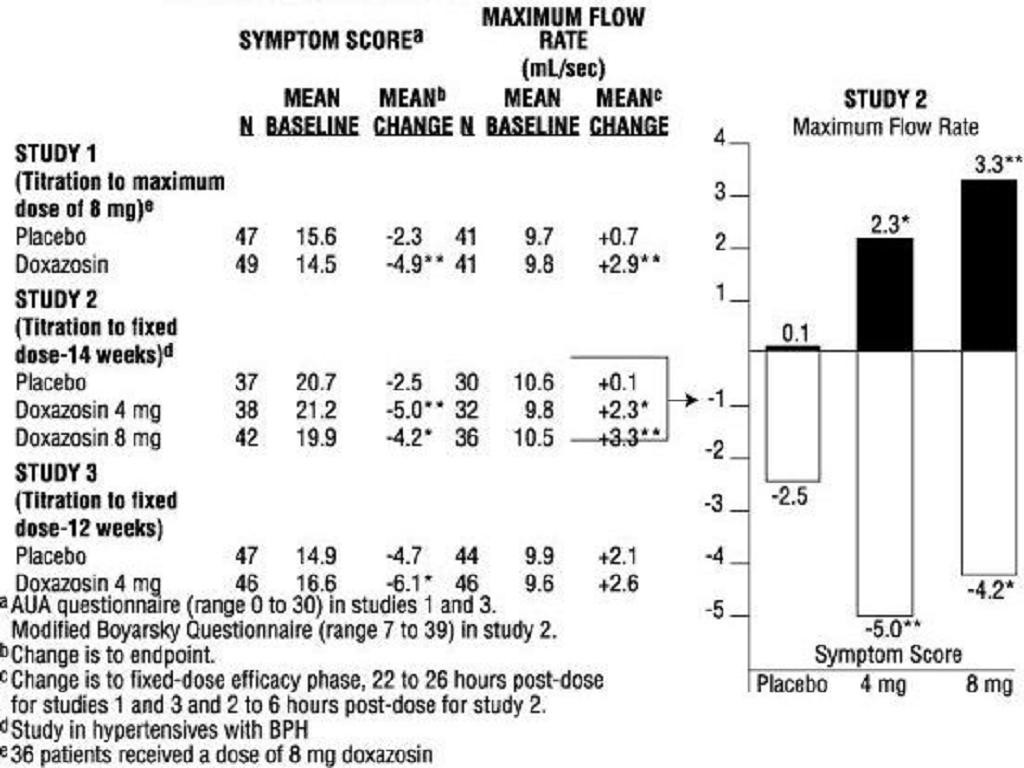
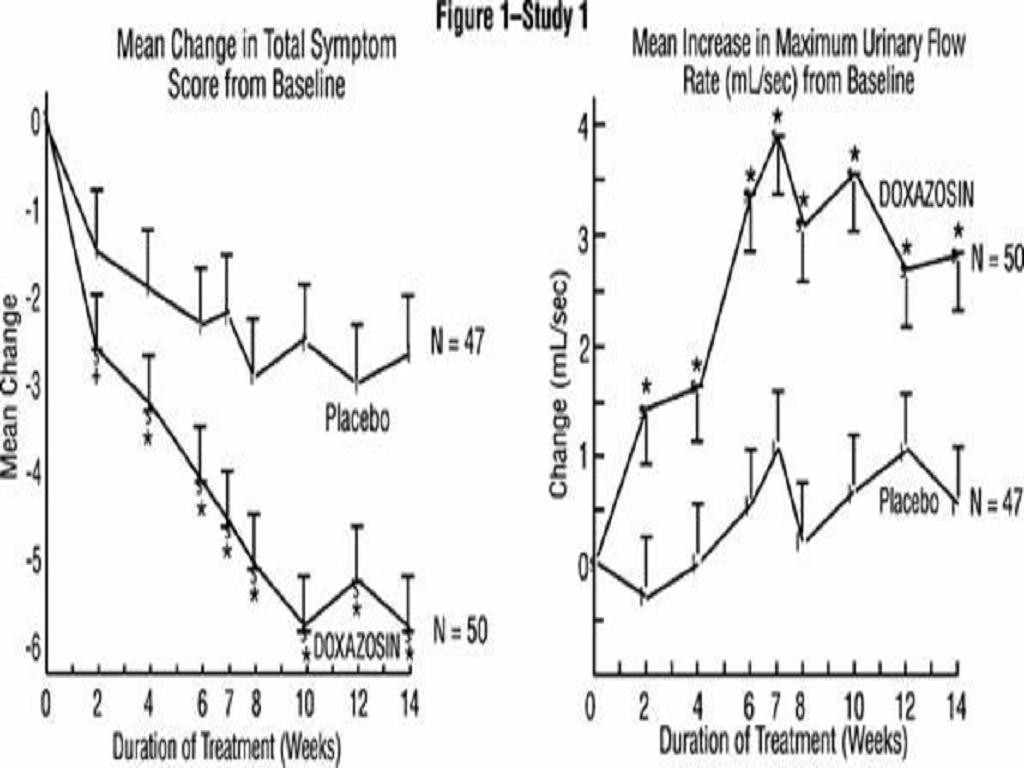
PLACEBODOXAZOSIN(N=85)(N=183)*****
Hypertension
Pharmacokinetics
PRECAUTIONS
INDICATIONS & USAGE
INDICATIONS AND USAGEBenign Prostatic Hyperplasia (BPH)
Hypertension
DOXAZOSIN MESYLATE CONTRAINDICATIONS
CONTRAINDICATIONSWARNINGS
Syncope and "First-dose" EffectDOSAGE AND ADMINISTRATION
Priapism
PRECAUTIONS: Information for Patients
PRECAUTIONS
GeneralProstate Cancer
Cataract Surgery
Orthostatic Hypotension
Hypertension
Benign Prostatic Hyperplasia
INFORMATION FOR PATIENTS
Information for PatientsPatient Leaflet
DRUG & OR LABORATORY TEST INTERACTIONS
Drug/Laboratory Test InteractionsImpaired Liver Function
CLINICAL PHARMACOLOGY
Leukopenia/Neutropenia
DRUG INTERACTIONS
Drug InteractionsCardiac Toxicity in Animals
PREGNANCY
Teratogenic EffectsPregnancy Category C
Nonteratogenic Effects
NURSING MOTHERS
Nursing MothersPEDIATRIC USE
Pediatric UseGERIATRIC USE
Geriatric UseDOXAZOSIN MESYLATE ADVERSE REACTIONS
Benign Prostatic HyperplasiaBENIGN PROSTATIC HYPERPLASIABody SystemDOXAZOSINPLACEBO(N=665)(N=300)BODY AS A WHOLE*CARDIOVASCULAR SYSTEM*DIGESTIVE SYSTEMMETABOLIC AND NUTRITIONAL DISORDERS*NERVOUS SYSTEM*RESPIRATORY SYSTEM*SPECIAL SENSESUROGENITAL SYSTEMSKIN & APPENDAGESPSYCHIATRIC DISORDERS*
Hypertension
HYPERTENSIONDOXAZOSINPLACEBO(N=339)(N=336)CARDIOVASCULAR SYSTEMSKIN & APPENDAGESMUSCULOSKELETAL SYSTEMCENTRAL & PERIPHERAL N.S.AUTONOMICSPECIAL SENSESPSYCHIATRICGASTROINTESTINALRESPIRATORYURINARYGENERAL
PRECAUTIONSPRECAUTIONS: General: Cataract Surgery
OVERDOSAGE
OVERDOSAGEDOSAGE & ADMINISTRATION
Benign Prostatic Hyperplasia 1 to 8 mg Once Daily
Hypertension 1 to 16 mg Once Daily
HOW SUPPLIED
STORAGE AND HANDLING
SPL PATIENT PACKAGE INSERT
PATIENT INFORMATION ABOUT DOXAZOSIN TABLETS, USPFOR BENIGN PROSTATIC HYPERPLASIA (BPH)
-
● before you start taking doxazosin.
-
● each time you get a new prescription.
-
● You and your doctor should discuss this treatment and your (BPH) symptoms before you start taking doxazosin and at your regular checkups. This leaflet does NOT take the place of discussions with your doctor.
-
● a feeling that the bladder is not completely emptied after urination
-
● a delay or difficulty in the beginning of urination
-
● a need to urinate often during the day and especially at night
-
● a feeling that you must urinate immediately.
-
● TREATMENT OPTIONS FOR BPH: The four main treatment options for BPH are:
-
● Treatment with doxazosin or other similar drugs. Doxazosin is the medication your doctor has prescribed for you. See "What Doxazosin Does", below.
-
● Treatment with the medication class of 5-alpha reductase inhibitors (e.g., finasteride). It can cause the prostate to shrink. It may take 6 months or more for the full benefit of finasteride to be seen.
-
● Various surgical procedures. Your doctor can describe these procedures to you. The best procedure for you depends on your BPH symptoms and medical condition.
-
● WHAT DOXAZOSIN DOES: Doxazosin works on a specific type of muscle found in the prostate, causing it to relax. This in turn decreases the pressure within the prostate, thus improving the flow of urine and your symptoms.
-
● If doxazosin is helping you, you should notice an affect within 1 to 2 weeks after you start your medication. Doxazosin has been studied in over 900 patients for up to 2 years and the drug has been shown to continue to work during long-term treatment. Even though you take doxazosin and it may help you, doxazosin may not prevent the need for surgery in the future.
-
● Doxazosin does not affect PSA levels. PSA is the abbreviation for Prostate Specific Antigen. Your doctor may have done a blood test called PSA. You may want to ask your doctor more about this if you have had a PSA test done.
-
● OTHER IMPORTANT FACTS:
-
● Doxazosin Mesylate is not a treatment for prostate cancer. Your doctor has prescribed doxazosin for your BPH and not for prostate cancer; however, a man can have BPH and prostate cancer at the same time. Doctors usually recommend that men be checked for prostate cancer once a year when they turn 50 (or 40 if a family member has had prostate cancer). A higher incidence of prostate cancer has been noted in men of African-American descent. These checks should continue even if you are taking doxazosin.
-
● HOW TO TAKE DOXAZOSIN AND WHAT YOU SHOULD KNOW WHILE TAKING DOXAZOSIN FOR BPH: Doxazosin Can Cause a Sudden Drop in Blood Pressure After the VERY FIRST DOSE. You may feel dizzy, faint or "light-headed", especially after you stand up from a lying or sitting position. This is more likely to occur after you've taken the first few doses or if you increase your dose, but can occur at any time while you are taking the drug. It can also occur if you stop taking the drug and then restart treatment. If you feel very dizzy, faint or "light-headed" you should contact your doctor. Your doctor will discuss with you how often you need to visit and how often your blood pressure should be checked.
-
● Other side effects you could have while taking doxazosin, in addition to lowering of the blood pressure, include dizziness, fatigue (tiredness), swelling of the feet and shortness of breath. Most side effects are mild. However, you should discuss any unexpected effects you notice with your doctor.
-
● WARNING: Extremely rarely, doxazosin and similar medications have caused painful erection of the penis, sustained for hours and unrelieved by sexual intercourse or masturbation. This condition is serious, and if untreated it can be followed by permanent inability to have an erection. If you have a prolonged abnormal erection, call your doctor or go to an emergency room as soon as possible.
-
● Keep doxazosin and all medicines out of the reach of children.
-
● FOR MORE INFORMATION ABOUT DOXAZOSIN AND BPH TALK WITH YOUR DOCTOR, NURSE, PHARMACIST OR OTHER HEALTH CARE PROVIDER.
INACTIVE INGREDIENT
INACTIVE INGREDIENTS:anhydrous lactose
colloidal silicon
magnesium stearate
microcrystalline cellulose
sodium lauryl sulfate
sodium starch glycolate
FD&C Blue No. 2 Aluminum Lake
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Doxazosin MesylateDoxazosin Mesylate TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!