Laser Pharmaceuticals, LLC
Donatussin DM DROPS
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
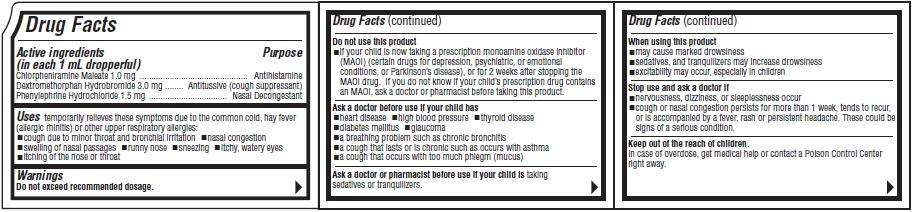
Active ingredients
(in each 1 mL dropperful)Purpose
Donatussin DM Drops Uses
- cough due to minor throat and bronchial irritation
- nasal congestion
- swelling of nasal passages
- runny nose
- sneezing
- itchy, water eyes
- itching of the nose or throat
Warnings
Do not exceed recommended dosage.Do not use this product
- if your child is now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if your child has
- heart disease
- high blood pressure
- thyroid disease
- diabetes mellitus
- glaucoma
- a breathing problem such as chronic bronchitis
- a cough that lasts or is chronic such as occurs with asthma
- a cough that occurs with too much phlegm (mucus)
Ask a doctor or pharmacist before use if your child is
When using this product
- may cause marked drowsiness
- sedatives, and tranquilizers may increase drowsiness
- excitability may occur, especially in children
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- cough or nasal congestion persists for more than 1 week, tends to recur, or is accompanied by a fever, rash or persistent headache. These could be signs of a serious condition.
Keep out of the reach of children.
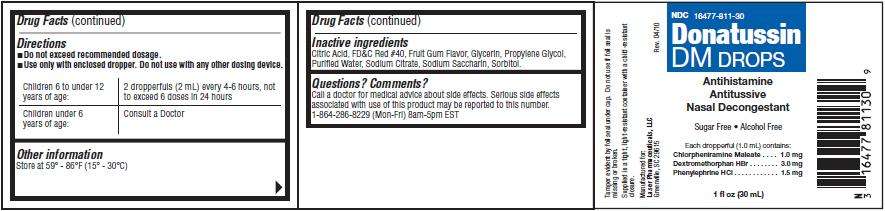
Directions
- Do not exceed recommended dosage.
- Use only with enclosed dropper. Do not use with any other dosing device.
Children 6 to under 12
years of age:
|
2 dropperfuls (2 mL) every 4-6 hours, not
to exceed 6 doses in 24 hours
|
Children under 6
years of age:
|
Consult a Doctor
|
Donatussin DM Drops Other information
Inactive ingredients
Questions? Comments?
PRODUCT PACKAGING:

Donatussin DM Drops
Chlorpheniramine Maleate, Dextromethorphan Hydrobromide, Phenylephrine Hydrochloride LIQUID
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:16477-811 |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
30 in 1 BOTTLE |
|
|
|
2 |
NDC:16477-811-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part341 |
2005-12-20 |
|
|


