DIPHENHYDRAMINE HYDROCHLORIDE
Humanwell PuraCap Pharmaceutical (Wuhan), Ltd.
DIPHENHYDRAMINE HYDROCHLORIDE 50 mg capsule, liquid filled
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each capsule)
- Purpose
- DIPHENHYDRAMINE HYDROCHLORIDE Uses
- Warnings
- Directions
- DIPHENHYDRAMINE HYDROCHLORIDE Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL - Shipping Label
- PRINCIPAL DISPLAY PANEL - Shipping Label
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient (in each capsule)
Diphenhydramine HCl 50 mg
Purpose
Nighttime sleep-aid
DIPHENHYDRAMINE HYDROCHLORIDE Uses
- for the relief of occasional sleeplessness
Warnings
- do not give to children under 12 years of age
- do not use with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers.
When using this product avoid alcoholic drinks.
Stop use and ask a doctor if
- sleeplessness persist continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years of age and over: 1 softgel (50 mg) at bedtime if needed, or as directed by a doctor.
DIPHENHYDRAMINE HYDROCHLORIDE Other information
- store at room temperature 15°-30°C (59°-86°F) and avoid excessive heat
- do not use if imprinted safety seal under cap is broken or missing
Inactive ingredients
FD&C blue #1, gelatin, glycerin, polyethylene glycol, purified water, sorbitol special and white edible ink.
Manufactured by:
Humanwell PuraCap Pharmaceutical (Wuhan) Ltd.
Wuhan, Hubei
430206, China
PRINCIPAL DISPLAY PANEL - Shipping Label
DIPHENHYDRAMINE HYDROCHLORIDE CAPSULES, 50 mg
Quantity : 12000 Capsules
NDC. No : 53345-005-01
IMPORTANT:
Inspect immediate upon receipt.
This is a bulk shipment intended for further processing only.
Protect from heat, humidity, and light. Do not refrigerate.
CAUTION : "FOR FURTHER MANUFACTURING, PROCESSING OR REPACKING"
 USP Bulk Label_f8de6605.jpg)
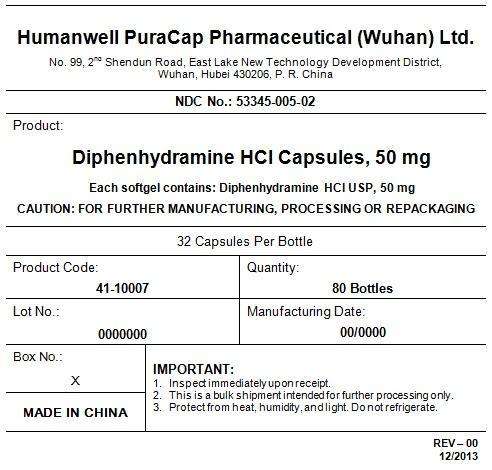
PRINCIPAL DISPLAY PANEL - Shipping Label
DIPHENHYDRAMINE HYDROCHLORIDE CAPSULES, 50 mg
Quantity : 80 Bottles
NDC. No : 53345-005-02
IMPORTANT:
Inspect immediate upon receipt.
This is a bulk shipment intended for further processing only.
Protect from heat, humidity, and light. Do not refrigerate.
CAUTION : "FOR FURTHER MANUFACTURING, PROCESSING OR REPACKING"
DIPHENHYDRAMINE HYDROCHLORIDEDIPHENHYDRAMINE HYDROCHLORIDE CAPSULE, LIQUID FILLED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||