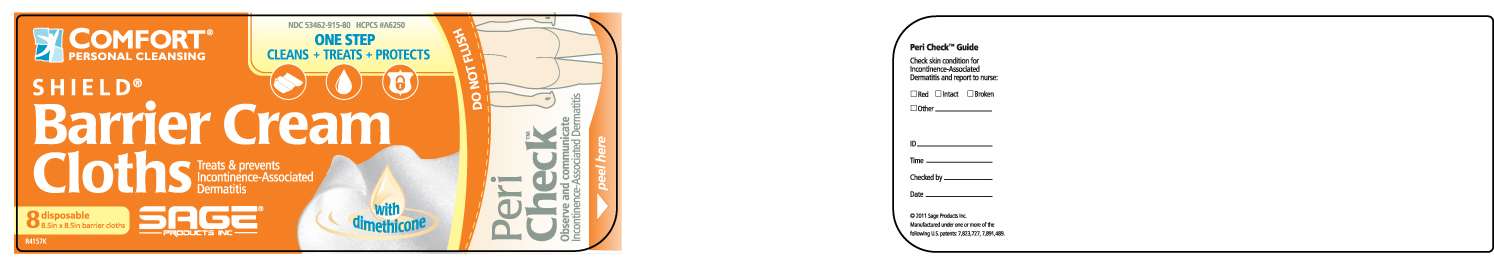
dimethicone
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Dimethicone 3%
Purpose
Skin protectant
If swallowed, get medical help or contact a Poison Control Center right away.
Uses
- helps seal out wetness
- helps treat and prevent perineal dermatitis
- protects from minor skin irritation associated with perineal dermatitis
Warnings
For external perineal use only. Avoid contact with the eyes.
Stop use and consult a doctor if condition worsens or does not improve within 7 days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- remove wet and soiled garment
- cleanse soiled area using a fresh barrier cloth
- use as often as necessary, especially if prolonged exposure to wetness is anticipated
- for individual use only
- discard immediately if barrier cloths are dry
water, glycerin, PPG-15 stearyl ether, glyceryl stearate, PEG-100 stearate, stearic acid, aloe barbadensis gel, stearyl alcohol, chlorhexidine gluconate, hydroxyethyl cellulose, methylparaben, propylparaben
barrier cloths: cellulose fiber/polyester (PET) fiber, 5.5" x 8.5" (small cloths), 8.5" x 8.5" (large cloths)

dimethiconedimethicone CLOTH
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||