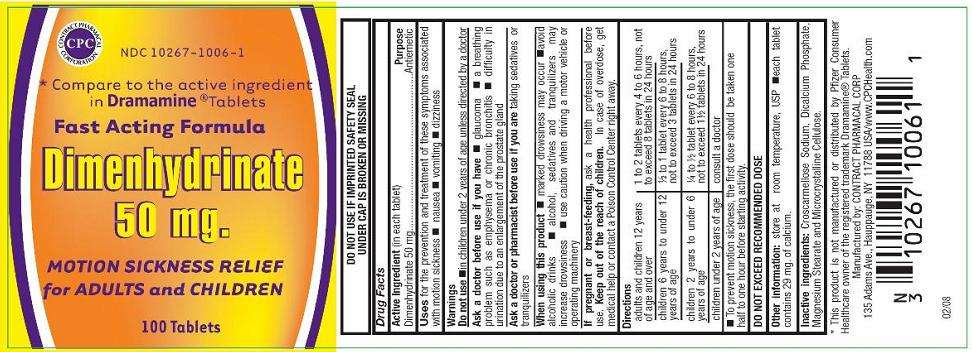
Dimenhydrinate
FULL PRESCRIBING INFORMATION
*Compare to the active ingredient in Dramamine® Tablets
Dimenhydrinate 50mg
MOTION SICKNESS RELIEF for ADULTS and CHILDREN
100 Tablets
DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Drug Facts
(in each tablet)
Dimenhydrinate 50 mg
Antiemetic
for prevention and treatment of these symptoms associated with motion sickness
- nausea
- vomiting
- dizziness
Do not use
- in children under 2 years of age unless directed by a doctor
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
taking sedatives or tranquilizers.
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives and tranquilizers may increase drowsiness
- use caution when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
ask a health care professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
| adults and children 12 years of age and over | 1 to 2 tablets every 4 to 6 hours, not to exceed 12 tablets in 24 hours |
| children 6 years to under 12 years of age | ½ to 1 tablet every 6 to 8 hours, not to exceed 3 tablets in 24 hours |
| children 2 years to under 6 years of age | ¼ to ½ tablet every 6 to 8 hours, not to exceed 1 ½ tablets in 24 hours |
| children under 2 years of age | consult a doctor |
- To prevent motion sickness, the first dose should be taken one half to one hour before starting activity.
DO NOT EXCEED RECOMMENDED DOSE
Other information: store at room temperature, USP
- Each tablet contains 29 mg of calcium
Croscarmellose Sodium, Dicalcium Phosphate, Magnesium Stearate and Microcrystalline Cellulose.
*This product is not manufactured or distributed by Pfizer Consumer Healthcare owner of the registered trademark Dramamine® Tablets.
Manufactured by: CONTRACT PHARMACAL CORP.
135 Adams Ave., Hauppauge, NY 11788 USA / www.CPCHealth.com
02/08
DimenhydrinateDimenhydrinate TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||