Diltiazem Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- DILTIAZEM HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- DILTIAZEM HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- DILTIAZEM HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
DILTIAZEM HYDROCHLORIDE DESCRIPTION
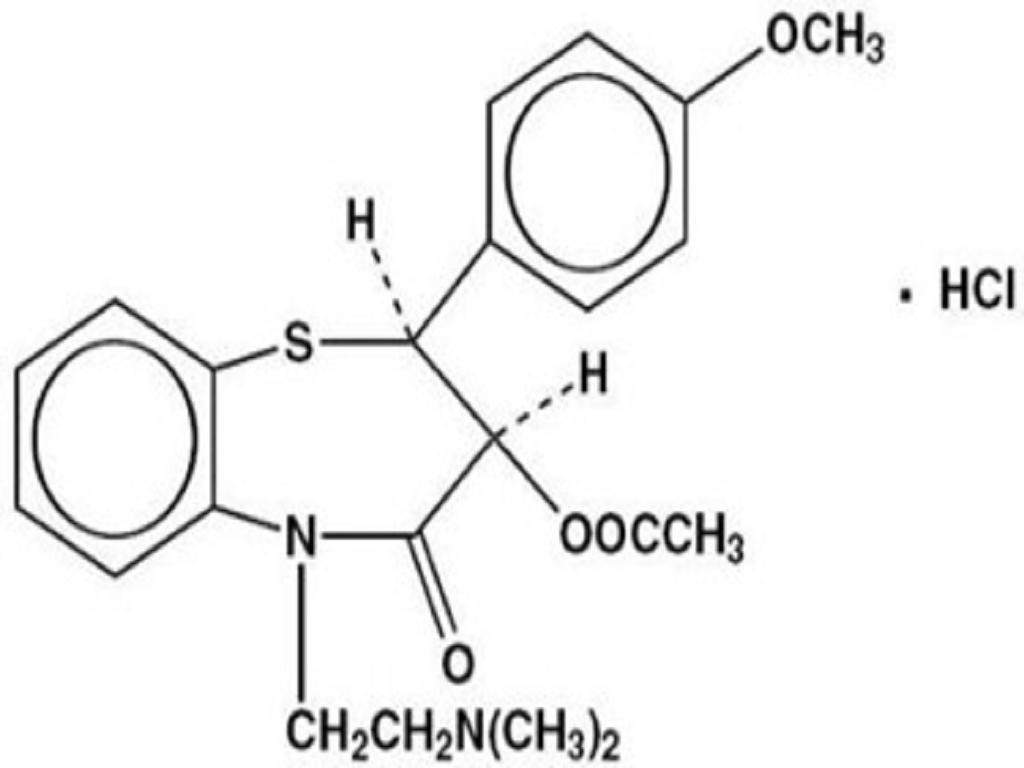
CLINICAL PHARMACOLOGY
Mechanisms of Action
1. Angina Due to Coronary Artery Spasm
2. Exertional Angina
Hemodynamic and Electrophysiologic Effects
Pharmacokinetics and Metabolism
INDICATIONS & USAGE
DILTIAZEM HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
WARNINGS1. Cardiac Conduction
ADVERSE REACTIONS
2. Congestive Heart Failure
3. Hypotension
4. Acute Hepatic Injury
PRECAUTIONS
PRECAUTIONS
GeneralADVERSE REACTIONS
DRUG INTERACTIONS
Drug InteractionsWARNINGS
WARNINGS
Beta-Blockers
WARNINGS
Cimetidine
Digitalis
WARNINGS
Anesthetics
Cyclosporine
Carbamazepine
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis, Impairment of FertilityPREGNANCY
Teratogenic EffectsPregnancy Category C
NURSING MOTHERS
PEDIATRIC USE
Pediatric UseDILTIAZEM HYDROCHLORIDE ADVERSE REACTIONS
ADVERSE REACTIONSconduction warning
hepatic warnings
OVERDOSAGE
OVERDOSAGE OR EXAGGERATED RESPONSEDOSAGE & ADMINISTRATION
Exertional Angina Pectoris Due to Atherosclerotic Coronary Artery Disease or Angina Pectoris at Rest Due to Coronary Artery SpasmConcomitant Use with Other Cardiovascular Agents
WARNINGSPRECAUTIONS
HOW SUPPLIED
STORAGE AND HANDLING
INACTIVE INGREDIENT
INACTIVE INGREDIENTethylcellulose
hypromellose
lactose monohydrate
magnesium stearate
maltodextrin
polyethylene glycol
sodium lauryl sulfate
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Diltiazem HydrochlorideDiltiazem Hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!