Diltiazem Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- DILTIAZEM HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS AND METABOLISM
- INDICATIONS & USAGE
- DILTIAZEM HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- DILTIAZEM HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
DILTIAZEM HYDROCHLORIDE DESCRIPTION
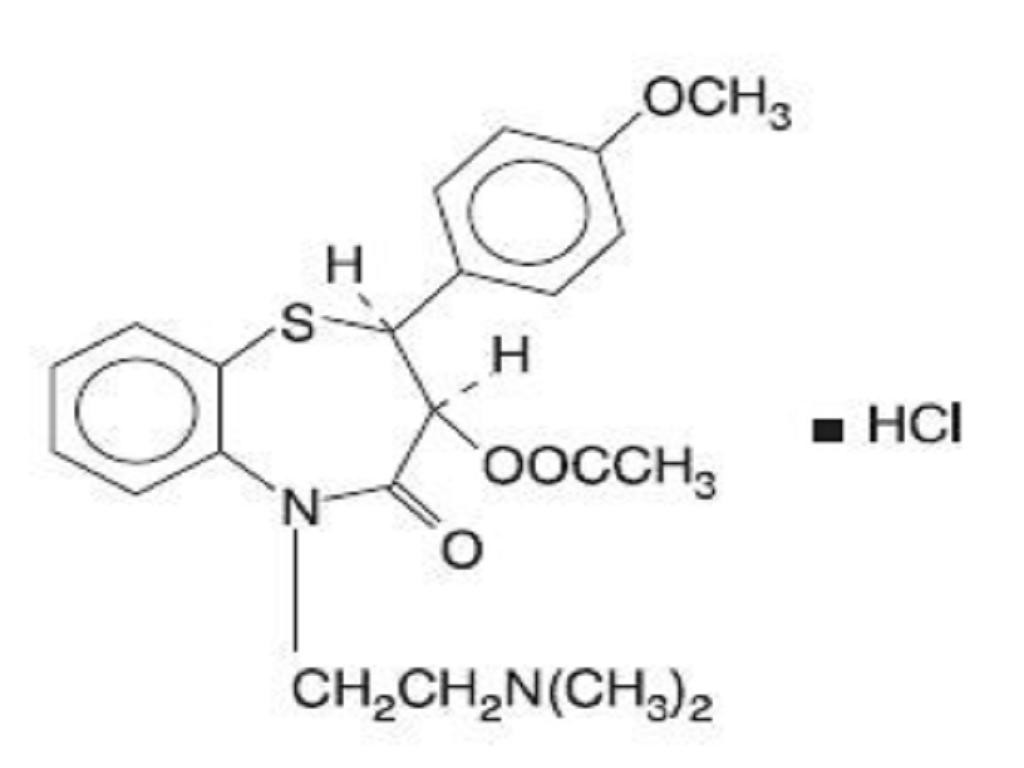
CLINICAL PHARMACOLOGY
Mechanisms of Action.
Hypertension:
Angina:
Hemodynamic and Electrophysiologic Effects.
WARNINGS
PHARMACODYNAMICS
Hypertension:Angina:
PHARMACOKINETICS AND METABOLISM
INDICATIONS & USAGE
Hypertension:Chronic Stable Angina:
DILTIAZEM HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
PRECAUTIONS
General.Dermatological eventsADVERSE REACTIONS
DRUG INTERACTIONS
WARNINGSWARNINGSWARNINGS
WARNINGS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
DILTIAZEM HYDROCHLORIDE ADVERSE REACTIONS
*
PlaceboDiltiazem hydrochloride extended-release capsulesAdverse Events (COSTART Term)n=57 # pts (%)Up to 360 mg n=149 # pts (%)480-540mg n=48 # pts (%)
*
PlaceboDiltiazem hydrochloride extended-release capsulesAdverse Events (COSTART Term)n=50 # pts (%)Up to 360 mg n=158 # pts (%)540 mg n=49 # pts (%)*
hepatic warnings
OVERDOSAGE
DOSAGE & ADMINISTRATION
Concomitant use with Other Cardiovascular Agents.
WARNINGSPRECAUTIONS
Sprinkling the Capsule Contents on Food
HOW SUPPLIED
StrengthDescriptionQuantityNDC#
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Diltiazem HydrochlorideDiltiazem Hydrochloride CAPSULE, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!