Diltiazem Hydrochloride
Diltiazem Hydrochloride Extended-Release Capsules, USP [Once-a-Day Dosage]
FULL PRESCRIBING INFORMATION: CONTENTS*
- DILTIAZEM HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- DILTIAZEM HYDROCHLORIDE INDICATIONS AND USAGE
- DILTIAZEM HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DILTIAZEM HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DILTIAZEM HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 120 MG
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 180 MG
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 240 MG
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 300 MG
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 360 MG
FULL PRESCRIBING INFORMATION
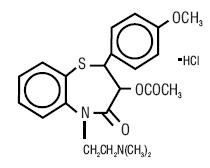
DILTIAZEM HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanisms of Action
Hypertension.
Angina.
Hemodynamic and Electrophysiologic Effects
WARNINGS
Pharmacokinetics and Metabolism
In vitroin vitro
Diltiazem Hydrochloride Extended-Release Capsules.
DILTIAZEM HYDROCHLORIDE INDICATIONS AND USAGE
DILTIAZEM HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
- Cardiac Conduction. Diltiazem prolongs AV node refractory periods without significantly prolonging sinus node recovery time, except in patients with sick sinus syndrome. This effect may rarely result in abnormally slow heart rates (particularly in patients with sick sinus syndrome) or second- or third-degree AV block (13 of 3290 patients or 0.40%). Concomitant use of diltiazem with beta-blockers or digitalis may result in additive effects on cardiac conduction. A patient with Prinzmetal's angina developed periods of asystole (2 to 5 seconds) after a single dose of 60 mg of diltiazem (see ADVERSE REACTIONS ).
- Congestive Heart Failure. Although diltiazem has a negative inotropic effect in isolated animal tissue preparations, hemodynamic studies in humans with normal ventricular function have not shown a reduction in cardiac index nor consistent negative effects on contractility (dp/dt). An acute study of oral diltiazem in patients with impaired ventricular function (ejection fraction 24% ± 6%) showed improvement in indices of ventricular function without significant decrease in contractile function (dp/dt). Worsening of congestive heart failure has been reported in patients with preexisting impairment of ventricular function. Experience with the use of diltiazem hydrochloride in combination with beta-blockers in patients with impaired ventricular function is limited. Caution should be exercised when using this combination.
- Hypotension. Decreases in blood pressure associated with diltiazem therapy may occasionally result in symptomatic hypotension.
- Acute Hepatic Injury. Mild elevations of transaminases with and without concomitant elevation in alkaline phosphatase and bilirubin have been observed in clinical studies. Such elevations were usually transient and frequently resolved even with continued diltiazem treatment. In rare instances, significant elevations in enzymes such as alkaline phosphatase, LDH, SGOT, SGPT, and other phenomena consistent with acute hepatic injury have been noted. These reactions tended to occur early after therapy initiation (1 to 8 weeks) and have been reversible upon discontinuation of drug therapy. The relationship to diltiazem is uncertain in some cases, but probable in some (see PRECAUTIONS ).
PRECAUTIONS
General
ADVERSE REACTIONS
Drug Interactions
WARNINGS WARNINGS
Anesthetics.
Benzodiazepines. max
Beta-blockers.
In vitro WARNINGS
Buspirone. max 1/2 max
Carbamazepine.
Cimetidine.
Clonidine.
Cyclosporine.
Digitalis. WARNINGS
Quinidine. (0 → ∞)1/2oral
Rifampin.
Statins.
maxmax
Carcinogenesis, Mutagenesis, Impairment of Fertility
in vitroin vivoin vitro
Pregnancy
Nursing Mothers
Pediatric Use
Geriatric Use
DILTIAZEM HYDROCHLORIDE ADVERSE REACTIONS
| Adverse Reactions | Diltiazem Hydrochloride Extended-Release Capsule (n=607) |
Placebo (n=301) |
|---|---|---|
| Headache |
5.4% |
5% |
| Dizziness |
3% |
3% |
| Bradycardia |
3.3% |
1.3% |
| AV Block First Degree |
3.3% |
0% |
| Edema |
2.6% |
1.3% |
| ECG Abnormality |
1.6% |
2.3% |
| Asthenia |
1.8% |
1.7% |
Cardiovascular:
Nervous System:
Gastrointestinal: WARNINGS, Acute Hepatic Injury
Dermatological:
Other:
OVERDOSAGE
505050
Bradycardia:
High-degree AV Block:
Cardiac Failure:
Hypotension:
DILTIAZEM HYDROCHLORIDE DOSAGE AND ADMINISTRATION
Hypertension.
Angina
Concomitant Use With Other Cardiovascular Agents
- Sublingual NTG. May be taken as required to abort acute anginal attacks during diltiazem hydrochloride therapy.
- Prophylactic Nitrate Therapy. Diltiazem hydrochloride extended-release capsules may be safely coadministered with short- and long-acting nitrates.
- Beta-blockers (see WARNINGS and PRECAUTIONS ).
- Antihypertensives. Diltiazem hydrochloride extended-release capsules have an additive antihypertensive effect when used with other antihypertensive agents. Therefore, the dosage of diltiazem hydrochloride extended-release capsules or the concomitant antihypertensives may need to be adjusted when adding one to the other.
HOW SUPPLIED
| Strength | Quantity | NDC Number | Description |
|---|---|---|---|
| 120 mg |
Bottle of 30 CRC Bottle of 90 CRC Bottle of 90 NCRC Bottle of 500 NCRC Bottle of 1000 NCRC |
47335-675-83 47335-675-81 47335-675-19 47335-675-13 47335-675-18 |
Hard gelatin capsules, size ‘2’Light turquoise blue colored cap and body, with “675” imprinted in black ink on cap and body, containing white to off white pellets. |
| 180 mg |
Bottle of 30 CRC Bottle of 90 CRC Bottle of 90 NCRC Bottle of 500 NCRC Bottle of 1000 NCRC |
47335-676-83 47335-676-81 47335-676-19 47335-676-13 47335-676-18 |
Hard gelatin capsules, size ‘0’ blue colored cap and light turquoise blue body, with “676” imprinted in black ink on cap and body, containing white to off white pellets. |
| 240 mg |
Bottle of 30 CRC Bottle of 90 CRC Bottle of 90 NCRC Bottle of 500 NCRC Bottle of 1000 NCRC |
47335-677-83 47335-677-81 47335-677-19 47335-677-13 47335-677-18 |
Hard gelatin capsules, size ‘0’ blue colored cap and blue colored body, with “677” imprinted in black ink on cap and body, containing white to off white pellets. |
| 300 mg |
Bottle of 30 CRC Bottle of 90 CRC Bottle of 90 NCRC Bottle of 500 NCRC Bottle of 1000 NCRC |
47335-678-83 47335-678-81 47335-678-19 47335-678-13 47335-678-18 |
Hard gelatin capsules, size ‘00’ blue colored cap and gray colored body, with “678” imprinted in black ink on cap and body, containing white to off white pellets. |
| 360 mg |
Bottle of 30 CRC Bottle of 90 CRC Bottle of 90 NCRC Bottle of 500 NCRC Bottle of 1000 NCRC |
47335-679-83 47335-679-81 47335-679-19 47335-679-13 47335-679-18 |
Hard gelatin capsules, size ‘00’ blue colored cap and white colored body with “679” imprinted in black ink on cap and body, containing white to off white pellets. |
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Ind. Ltd.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 120 MG
NDC 47335-675-83
Diltiazem Hydrochloride Extended-Release Capsules, USP
(Once-a-Day Dosage)
120 mg
Rx only
30 CAPSULES
SUN PHARMACEUTICAL INDUSTRIES LTD.

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 180 MG
NDC 47335-676-83
Diltiazem Hydrochloride Extended-Release Capsules, USP
(Once-a-Day Dosage)
180 mg
Rx only
30 CAPSULES
SUN PHARMACEUTICAL INDUSTRIES LTD.

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 240 MG
NDC 47335-677-83
Diltiazem Hydrochloride Extended-Release Capsules, USP
(Once-a-Day Dosage)
240 mg
Rx only
30 CAPSULES
SUN PHARMACEUTICAL INDUSTRIES LTD.

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 300 MG
NDC 47335-678-83
Diltiazem Hydrochloride Extended-Release Capsules, USP
(Once-a-Day Dosage)
300 mg
Rx only
30 CAPSULES
SUN PHARMACEUTICAL INDUSTRIES LTD.

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 360 MG
NDC 47335-679-83
Diltiazem Hydrochloride Extended-Release Capsules, USP
(Once-a-Day Dosage)
360 mg
Rx only
30 CAPSULES
SUN PHARMACEUTICAL INDUSTRIES LTD.

Diltiazem HydrochlorideDiltiazem Hydrochloride CAPSULE, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Diltiazem HydrochlorideDiltiazem Hydrochloride CAPSULE, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Diltiazem HydrochlorideDiltiazem Hydrochloride CAPSULE, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Diltiazem HydrochlorideDiltiazem Hydrochloride CAPSULE, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Diltiazem HydrochlorideDiltiazem Hydrochloride CAPSULE, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!