Diltiazem Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- DILTIAZEM HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- DILTIAZEM HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- DILTIAZEM HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
DILTIAZEM HYDROCHLORIDE DESCRIPTION
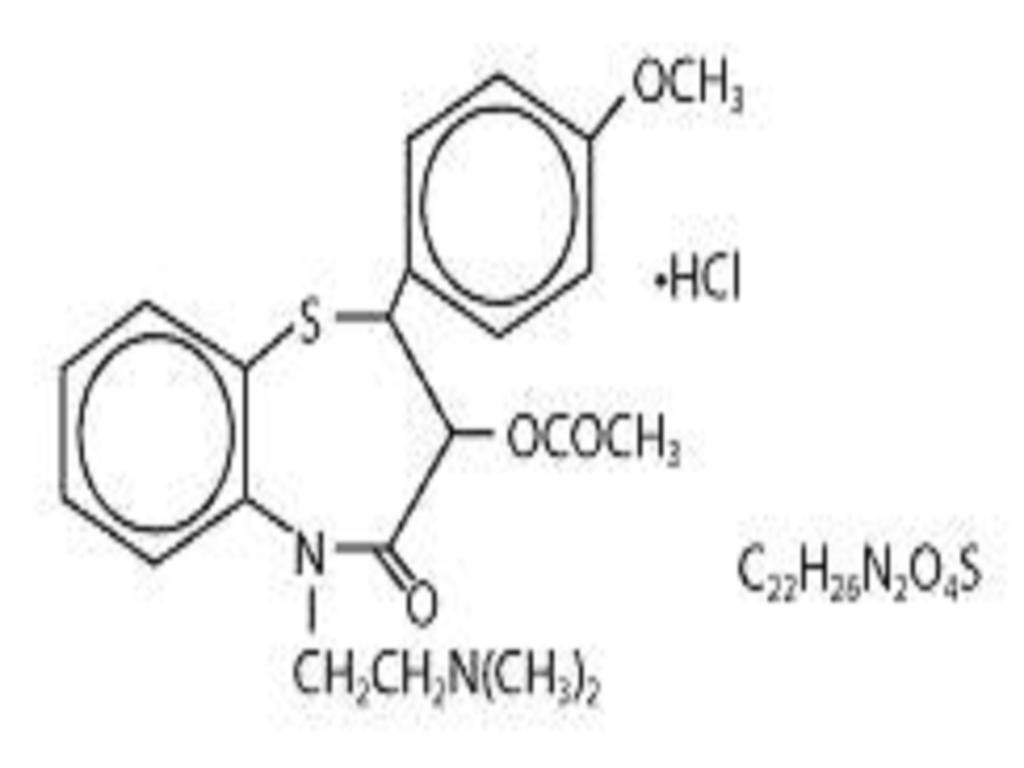
CLINICAL PHARMACOLOGY
Mechanism of Action
Hemodynamic and Electrophysiologic Effects
WARNINGS
Pharmacokinetics and Metabolism
INDICATIONS & USAGE
DILTIAZEM HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
ADVERSE REACTIONSPRECAUTIONS
PRECAUTIONS
GeneralADVERSE REACTIONS
DRUG INTERACTIONS
WARNINGSWARNINGSWARNINGS
WARNINGS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
DILTIAZEM HYDROCHLORIDE ADVERSE REACTIONS
WARNINGS, Acute Hepatic Injury
OVERDOSAGE
DOSAGE & ADMINISTRATION
WARNINGSPRECAUTIONS
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Diltiazem HydrochlorideDiltiazem Hydrochloride CAPSULE, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!