Diltiazem Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- DILTIAZEM HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS AND METABOLISM
- INDICATIONS & USAGE
- DILTIAZEM HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- DILTIAZEM HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
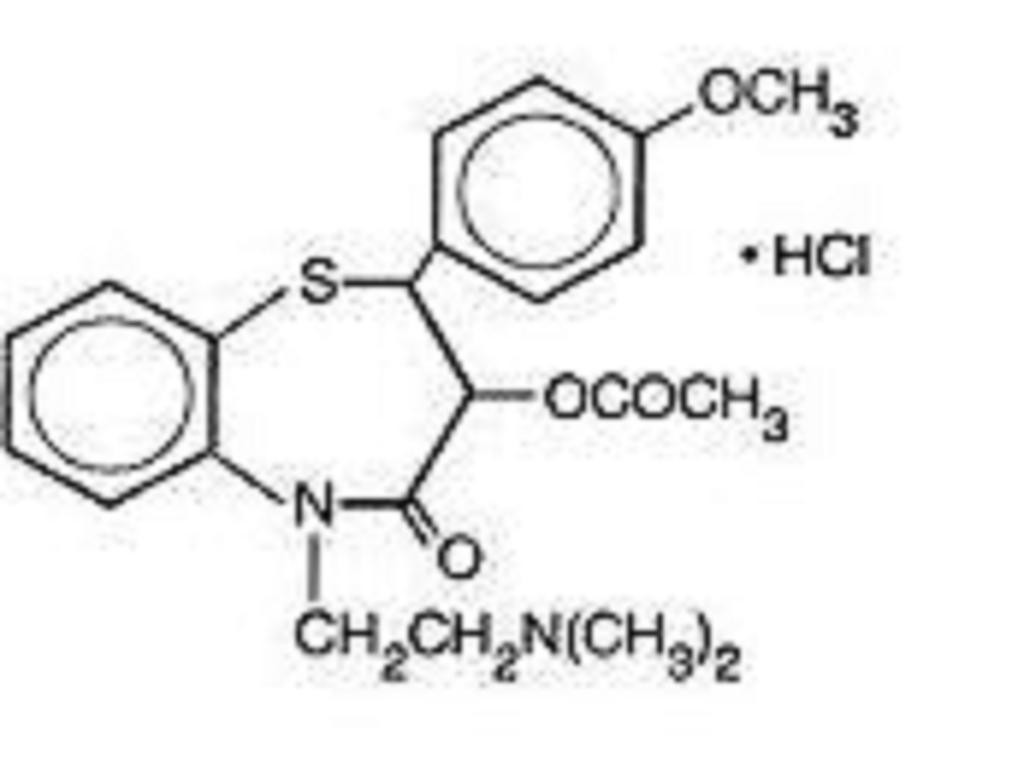
DILTIAZEM HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanisms of Action
Angina Due to Coronary Artery Spasm:
Exertional Angina:
Hemodynamic and Electrophysiologic Effects
PHARMACOKINETICS AND METABOLISM
Diltiazem Tablets
INDICATIONS & USAGE
DILTIAZEM HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
1.Cardiac Conduction:2.Congestive Heart Failure:
3.Hypotension:
4.Acute Hepatic Injury:
PRECAUTIONS
GeneralDRUG INTERACTIONS
Anesthetics
Benzodiazepines
Beta-Blockers
Buspirone
Carbamazepine
Cimetidine
Clonidine
Cyclosporine
Digitalis
Quinidine
Rifampin
Statins
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy category C
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
DILTIAZEM HYDROCHLORIDE ADVERSE REACTIONS
Cardiovascular:
Nervous System:
Gastrointestinal:
Dermatological:
Other:
OVERDOSAGE
Bradycardia:
High-Degree AV Block:
Cardiac Failure:
Hypotension
DOSAGE & ADMINISTRATION
Exertional Angina Pectoris Due to Atherosclerotic Coronary Artery Disease or Angina Pectoris at Rest Due to Coronary Artery SpasmConcomitant Use With Other Cardiovascular Agents
Sublingual NTG
Prophylactic Nitrate Therapy:
Beta-blockers.
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Diltiazem HydrochlorideDiltiazem Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!