DIFFERIN
DIFFERIN (adapalene gel)Gel, 0.1%Rx Only
FULL PRESCRIBING INFORMATION: CONTENTS*
- DIFFERIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- DIFFERIN INDICATIONS AND USAGE
- DIFFERIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DIFFERIN ADVERSE REACTIONS
- OVERDOSAGE
- DIFFERIN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL
FULL PRESCRIBING INFORMATION
DIFFERIN DESCRIPTION
DIFFERIN® Gel, containing adapalene, is used for the topical treatment of acne vulgaris. Each gram of DIFFERIN Gel contains adapalene 0.1% (1 mg) in a vehicle consisting of carbomer 940, edetate disodium, methylparaben, poloxamer 182, propylene glycol, purified water and sodium hydroxide. May contain hydrochloric acid to adjust pH.
The chemical name of adapalene is 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid. Adapalene is a white to off-white powder which is soluble in tetrahydrofuran, sparingly soluble in ethanol, and practically insoluble in water. The molecular formula is C28H28O3 and molecular weight is 412.52. Adapalene is represented by the following structural formula:
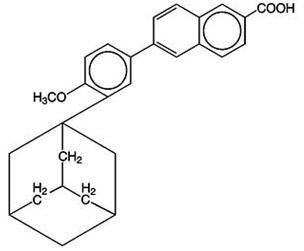
CLINICAL PHARMACOLOGY
Adapalene is a chemically stable, retinoid-like compound. Biochemical and pharmacological profile studies have demonstrated that adapalene is a modulator of cellular differentiation, keratinization, and inflammatory processes all of which represent important features in the pathology of acne vulgaris.
Mechanistically, adapalene binds to specific retinoic acid nuclear receptors but does not bind to the cytosolic receptor protein. Although the exact mode of action of adapalene is unknown, it is suggested that topical adapalene may normalize the differentiation of follicular epithelial cells resulting in decreased microcomedone formation.
Absorption of adapalene through human skin is low. Only trace amounts (<0.25 ng/mL) of parent substance have been found in the plasma of acne patients following chronic topical application of adapalene in controlled clinical trials. Excretion appears to be primarily by the biliary route.
DIFFERIN INDICATIONS AND USAGE
DIFFERIN Gel is indicated for the topical treatment of acne vulgaris.
DIFFERIN CONTRAINDICATIONS
DIFFERIN Gel should not be administered to individuals who are hypersensitive to adapalene or any of the components in the vehicle gel.
WARNINGS
Use of DIFFERIN Gel should be discontinued if hypersensitivity to any of the ingredients is noted. Patients with sunburn should be advised not to use the product until fully recovered.
PRECAUTIONS
If a reaction suggesting sensitivity or chemical irritation occurs, use of the medication should be discontinued. Exposure to sunlight, including sunlamps, should be minimized during the use of adapalene. Patients who normally experience high levels of sun exposure, and those with inherent sensitivity to sun, should be warned to exercise caution. Use of sunscreen products and protective clothing over treated areas is recommended when exposure cannot be avoided. Weather extremes, such as wind or cold, also may be irritating to patients under treatment with adapalene.
Avoid contact with the eyes, lips, angles of the nose, and mucous membranes. The product should not be applied to cuts, abrasions, eczematous skin, or sunburned skin.
Certain cutaneous signs and symptoms such as erythema, dryness, scaling, burning, or pruritus may be experienced during treatment. These are most likely to occur during the first two to four weeks and will usually lessen with continued use of the medication. Depending upon the severity of adverse events, patients should be instructed to reduce the frequency of application or discontinue use.
As DIFFERIN Gel has the potential to produce local irritation in some patients, concomitant use of other potentially irritating topical products (medicated or abrasive soaps and cleansers, soaps and cosmetics that have a strong drying effect, and products with high concentrations of alcohol, astringents, spices, or lime) should be approached with caution. Particular caution should be exercised in using preparations containing sulfur, resorcinol, or salicylic acid in combination with DIFFERIN Gel. If these preparations have been used, it is advisable not to start therapy with DIFFERIN Gel until the effects of such preparations in the skin have subsided.
Carcinogenicity studies with adapalene have been conducted in mice at topical doses of 0.3, 0.9, and 2.6 mg/kg/day and in rats at oral doses of 0.15, 0.5, and 1.5 mg/kg/day, approximately 4-75 times the maximal daily human topical dose. In the oral study, positive linear trends were observed in the incidence of follicular cell adenomas and carcinomas in the thyroid glands of female rats, and in the incidence of benign and malignant pheochromocytomas in the adrenal medullas of male rats.
No photocarcinogenicity studies were conducted. Animal studies have shown an increased tumorigenic risk with the use of pharmacologically similar drugs (e.g., retinoids) when exposed to UV irradiation in the laboratory or to sunlight. Although the significance of these studies to human use is not clear, patients should be advised to avoid or minimize exposure to either sunlight or artificial UV irradiation sources.
In a series of in vivo and in vitro studies, adapalene did not exhibit mutagenic or genotoxic activities.
Teratogenic Effects. Pregnancy Category C. No teratogenic effects were seen in rats at oral doses of adapalene 0.15 to 5.0 mg/kg/day, up to 120 times the maximal daily human topical dose. Cutaneous route teratology studies conducted in rats and rabbits at doses of 0.6, 2.0, and 6.0 mg/kg/day, up to 150 times the maximal daily human topical dose exhibited no fetotoxicity and only minimal increases in supernumerary ribs in rats. There are no adequate and well-controlled studies in pregnant women. Adapalene should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when DIFFERIN Gel is administered to a nursing woman.
Safety and effectiveness in pediatric patients below the age of 12 have not been established.
DIFFERIN ADVERSE REACTIONS
Some adverse effects such as erythema, scaling, dryness, pruritus, and burning will occur in 10-40% of patients. Pruritus or burning immediately after application also occurs in approximately 20% of patients. The following additional adverse experiences were reported in approximately 1% or less of patients: skin irritation, burning/stinging, erythema, sunburn, and acne flares. These are most commonly seen during the first month of therapy and decrease in frequency and severity thereafter. All adverse effects with use of DIFFERIN Gel during clinical trials were reversible upon discontinuation of therapy.
OVERDOSAGE
DIFFERIN Gel is intended for cutaneous use only. If the medication is applied excessively, no more rapid or better results will be obtained and marked redness, peeling, or discomfort may occur. The acute oral toxicity of DIFFERIN Gel in mice and rats is greater than 10 mL/kg. Chronic ingestion of the drug may lead to the same side effects as those associated with excessive oral intake of Vitamin A.
DIFFERIN DOSAGE AND ADMINISTRATION
DIFFERIN Gel should be applied once a day to affected areas after washing in the evening before retiring. A thin film of the gel should be applied, avoiding eyes, lips, and mucous membranes.
During the early weeks of therapy, an apparent exacerbation of acne may occur. This is due to the action of the medication on previously unseen lesions and should not be considered a reason to discontinue therapy. Therapeutic results should be noticed after eight to twelve weeks of treatment.
HOW SUPPLIED
DIFFERIN (adapalene gel) Gel, 0.1% is supplied in the following size:
45 g laminate tube - NDC 0299-5910-45
Store at controlled room temperature 20° - 25°C (68° - 77°F).
Marketed by:
GALDERMA LABORATORIES, L.P.
Fort Worth, Texas 76177 USA
Mfd. by:
DPT Laboratories, Ltd.
San Antonio, TX 78215
Mfd. by:
Galderma Production Canada, Inc.
Baie d’Urfe, QC, H9X 3S4 Canada
Made in Canada.
GALDERMA is a registered trademark.
325034-0903
P50045-0
Revised: September 2003
PACKAGE LABEL
Differin
®
(adapalene gel)
GEL
0.1%
NET WT. 45 g
NDC 0299-5910-45
Rx Only
GALDERMA
For External Use Only. Not For Ophthalmic Use. Store at controlled room temperature 68° to 77°F (20°-25°C).
Usual dosage: Apply a thin film once a day to affected areas after washing in the evening before retiring. See package insert for complete prescribing information.
Each gram contains: adapalene 0.1% (1 mg) in a vehicle consisting of carbomer 940, edetate disodium, methylparaben, poloxamer 182, propylene glycol, purified water, and sodium hydroxide. May contain hydrochloric acid to adjust pH.
Marketed by:
GALDERMA LABORATORIES, L.P.
Fort Worth, Texas 76177 USA
Manufactured by:
DPT Laboratories, Ltd.
San Antonio, Texas 78215 USA
GALDERMA is a registered trademark.
www.differin.com
310118-0306
Differin® (adapalene gel)
GEL
0.1%
NET WT. 45 g
NDC 0299-5910-45
Rx Only
GALDERMA
For External Use Only. Not For Ophthalmic Use. Store at controlled room temperature 68° to 77° (20°-25°C).
Usual dosage: Apply a thin film once a day to affected areas after washing in the evening before retiring. See package insert for complete prescribing information.
Each gram contains: adapalene 0.1% (1 mg) in a vehicle consisting of carbomer 940, edetate disodium, methylparaben, poloxamer 182, propylene glycol, purified water, and sodium hydroxide. May contain hydrochloric acid to adjust pH.
Marketed by:
GALDERMA LABORATORIES, L.P.
Fort Worth, Texas 76177 USA
Manufactured by:
Galderma Production Canada Inc.
Baie d’Urfe, QC, H9X 3S4 Canada
Made in Canada.
GALDERMA is a registered trademark.
P50053-2 www.differin.com
DIFFERINadapalene GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||