Desmopressin Acetate
Sun Pharmaceutical Industries Limited
FULL PRESCRIBING INFORMATION: CONTENTS*
- DESMOPRESSIN ACETATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- DESMOPRESSIN ACETATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DESMOPRESSIN ACETATE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
DESMOPRESSIN ACETATE DESCRIPTION
466414122
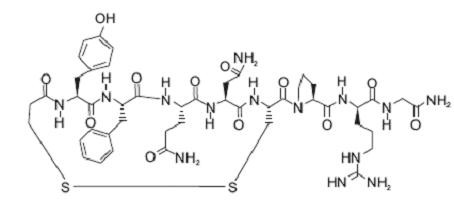
CLINICAL PHARMACOLOGY
- The biphasic half-lives of desmopressin acetate were 7.8 and 75.5 minutes for the fast and slow phases, respectively, compared with 2.5 and 14.5 minutes for lysine vasopressin, another form of the hormone. As a result, desmopressin acetate provides a prompt onset of antidiuretic action with a long duration after each administration.
- The change in structure of arginine vasopressin to desmopressin acetate has resulted in a decreased vasopressor action and decreased actions on visceral smooth muscle relative to the enhanced antidiuretic activity, so that clinically effective antidiuretic doses are usually below threshold levels for effects on vascular or visceral smooth muscle.
- When administered by injection, desmopressin acetate has an antidiuretic effect about ten times that of an equivalent dose administered intranasally.
- The bioavailability of the subcutaneous route of administration was determined qualitatively using urine output data. The exact fraction of drug absorbed by that route of administration has not been quantitatively determined.
- The percentage increase of factor VIII levels in patients with mild hemophilia A and von Willebrand’s disease was not significantly different from that observed in normal healthy individuals when treated with 0.3 mcg/kg of desmopressin acetate infused over 10 minutes.
- Plasminogen activator activity increases rapidly after desmopressin acetate infusion, but there has been no clinically significant fibrinolysis in patients treated with desmopressin acetate.
- The effect of repeated desmopressin acetate administration when doses were given every 12 to 24 hours has generally shown a gradual diminution of the factor VIII activity increase noted with a single dose.The initial response is reproducible in any particular patient if there are 2 or 3 days between administrations.
Human Pharmacokinetics: Desmopressin acetate is mainly excreted in the urine. A pharmacokinetic study conducted in healthy volunteers and patients with mild, moderate, and severe renal impairment (n=24, 6 subjects in each group) receiving single dose desmopressin acetate (2 mcg) injection demonstrated a difference in desmopressin acetate terminal half-life. Terminal half-life significantly increased from 3 hours in normal healthy patients to 9 hours in patients with severe renal impairment. (See CONTRAINDICATIONS .)
INDICATIONS & USAGE
Hemophilia A: Desmopressin Acetate Injection
| Desmopressin acetate injection is not indicated for the treatment of hemophilia A with factor VIII coagulant activity levels equal to or less than 5%, or for the treatment of hemophilia B, or in patients who have factor VIII antibodies. |
von Willebrand’s Disease (Type I): Desmopressin acetate injection
See WARNINGS.
Diabetes Insipidus: Desmopressin acetate injection
DESMOPRESSIN ACETATE CONTRAINDICATIONS
Desmopressin acetate injection .
WARNINGS
- Very rare cases of hyponatremia have been reported from world-wide postmarketing experience in patients treated with desmopressin acetate. Desmopressin acetate is a potent antidiuretic which, when administered, may lead to water intoxication and/or hyponatremia. Unless properly diagnosed and treated hyponatremia can be fatal. Therefore, fluid restriction is recommended and should be discussed with the patient and/or guardian. Careful medical supervision is required.
- When desmopressin acetate injection is administered to patients who do not have need of antidiuretic hormone for its antidiuretic effect, in particular in pediatric and geriatric patients, fluid intake should be adjusted downward to decrease the potential occurrence of water intoxication and hyponatremia. (See PRECAUTIONS , Pediatric Use and Geriatric Use.) All patients receiving desmopressin acetate therapy should be observed for the following signs of symptoms associated with hyponatremia: headache, nausea/vomiting, decreased serum sodium, weight gain, restlessness, fatigue, lethargy, disorientation, depressed reflexes, loss of appetite, irritability, muscle weakness, muscle spasms or cramps and abnormal mental status such as hallucinations, decreased consciousness and confusion. Severe symptoms may include one or a combination of the following: seizure, coma and/or respiratory arrest. Particular attention should be paid to the possibility of the rare occurrence of an extreme decrease in plasma osmolality that may result in seizures which could lead to coma.
- Desmopressin acetate should not be used to treat patients with Type IIB von Willebrand’s disease since platelet aggregation may be induced.
- Desmopressin acetate should be used with caution in patients with habitual or psychogenic polydipsia who may be more likely to drink excessive amounts of water, putting them at greater risk of hyponatremia.
PRECAUTIONS
General Precautions
Desmopressin acetate injection
desmopressin acetate injection
desmopressin acetate injection
Hemophilia A:
von Willebrand’s Disease:
Diabetes Insipidus:
Drug Interactions
Carcinogenesis & Mutagenesis & Impairment Of Fertility
Pregnancy
Pregnancy Category B2
Nursing Mothers
Pediatric Use
See WARNINGS Desmopressin acetateinjection should not be used in infants less than three months of ageGeriatric Use
See CLINICAL PHARMACOLOGY CONTRAINDICATIONS
See WARNINGS
DESMOPRESSIN ACETATE ADVERSE REACTIONS
Desmopressin acetateinjection desmopressin acetate injection
WARNINGS
Post Marketing
desmopressin acetate injectionOVERDOSAGE
See WARNINGS
desmopressin acetate injection
50
DOSAGE & ADMINISTRATION
Hemophilia A and von Willebrand’s Disease (Type I): Desmopressin acetate injection desmopressin acetate injection
See WARNINGS, PRECAUTIONS Pediatric UseGeriatric Use
Diabetes Insipidus: Desmopressin acetate injection
See WARNINGS, PRECAUTIONS Pediatric UseGeriatric Use
Geriatric UseSee CLINICAL PHARMACOLOGY CONTRAINDICATIONS PRECAUTIONS Geriatric Use
HOW SUPPLIED
Desmopressin acetate injection, USP
Keep out of the reach of children.
Sun Pharmaceutical Ind. Ltd.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 62756-529-40
Desmopressin Acetate Injection, USP
40 mcg/10 mL
(4 mcg/mL)
FOR INTRAVENOUS/SUBCUTANEOUS USE ONLY
Refrigerate
Rx only
One 10 mL Multiple-dose Vial
SUN PHARMA

Desmopressin AcetateDesmopressin Acetate INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!