Desipramine Hydrochloride
Bryant Ranch Prepack
Bryant Ranch Prepack
Desipramine Hydrochloride Tablets, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- DESIPRAMINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- DESIPRAMINE HYDROCHLORIDE INDICATIONS AND USAGE
- DESIPRAMINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DESIPRAMINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DESIPRAMINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- MEDICATION GUIDE
- Desipramine 25mg Tablet
FULL PRESCRIBING INFORMATION
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of desipramine hydrochloride tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Desipramine hydrochloride is not approved for use in pediatric patients. (See , , and .) WARNINGS: Clinical Worsening and Suicide Risk PRECAUTIONS: Information for Patients PRECAUTIONS: Pediatric Use
DESIPRAMINE HYDROCHLORIDE DESCRIPTION
Desipramine hydrochloride USP is an antidepressant drug of the tricyclic type available as tablets of 10 mg, 25 mg, 50 mg, 75 mg, 100 mg or 150 mg for oral administration. Its chemical name is 5 -Dibenz[ ]azepine-5-propanamine, 10, 11-dihydro- -methyl-, monohydrochloride. H bf N
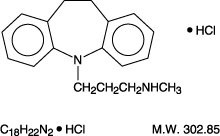
Inactive ingredients: hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, starch (corn), and titanium dioxide.
CLINICAL PHARMACOLOGY
DESIPRAMINE HYDROCHLORIDE INDICATIONS AND USAGE
Desipramine hydrochloride is indicated for the treatment of depression.
DESIPRAMINE HYDROCHLORIDE CONTRAINDICATIONS
Desipramine hydrochloride should not be given in conjunction with, or within 2 weeks of, treatment with an MAO inhibitor drug; hyperpyretic crises, severe convulsions, and death have occurred in patients taking MAO inhibitors and tricyclic antidepressants. When desipramine hydrochloride is substituted for an MAO inhibitor, at least 2 weeks should elapse between treatments. Desipramine hydrochloride should then be started cautiously and should be increased gradually.
Desipramine hydrochloride is contraindicated in the acute recovery period following myocardial infarction. It should not be used in those who have shown prior hypersensitivity to the drug. Cross-sensitivity between this and other dibenzazepines is a possibility.
WARNINGS
Use in Pregnancy
Safe use of desipramine hydrochloride during pregnancy and lactation has not been established; therefore, if it is to be given to pregnant patients, nursing mothers, or women of childbearing potential, the possible benefits must be weighed against the possible hazards to mother and child. Animal reproductive studies have been inconclusive.
Geriatric Use
Clinical studies of desipramine hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Lower doses are recommended for elderly patients. (See .) DOSAGE AND ADMINISTRATION
The ratio of 2-hydroxydesipramine to desipramine may be increased in the elderly, most likely due to decreased renal elimination with aging.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Desipramine hydrochloride use in the elderly has been associated with a proneness to falling as well as confusional states. (See .) ADVERSE REACTIONS
PRECAUTIONS
Information for Patients
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with desipramine hydrochloride and should counsel them in its appropriate use. A patient Medication Guide about “Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or Actions” is available for desipramine hydrochloride. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking desipramine hydrochloride.
Pediatric Use
Safety and effectiveness in the pediatric population have not been established (see and ). Therefore, desipramine hydrochloride is not recommended for use in children. BOX WARNING : WARNINGS Clinical Worsening and Suicide Risk
Anyone considering the use of desipramine hydrochloride in a child or adolescent must balance the potential risks with the clinical need (see also ). : ADVERSE REACTIONS Cardiovascular
General
It is important that this drug be dispensed in the least possible quantities to depressed outpatients, since suicide has been accomplished with this class of drug (see ). Ordinary prudence requires that children not have access to this drug or to potent drugs of any kind; if possible, this drug should be dispensed in containers with child-resistant safety closures. Storage of this drug in the home must be supervised responsibly. : WARNINGS Clinical Worsening and Suicide Risk
If serious adverse effects occur, dosage should be reduced or treatment should be altered. Desipramine hydrochloride therapy in patients with manic-depressive illness may induce a hypomanic state after the depressive phase terminates.
The drug may cause exacerbation of psychosis in schizophrenic patients.
Both elevation and lowering of blood sugar levels have been reported.
Leukocyte and differential counts should be performed in any patient who develops fever and sore throat during therapy; the drug should be discontinued if there is evidence of pathologic neutrophil depression.
Clinical experience in the concurrent administration of ECT and antidepressant drugs is limited. Thus, if such treatment is essential, the possibility of increased risk relative to benefits should be considered.
This drug should be discontinued as soon as possible prior to elective surgery because of possible cardiovascular effects. Hypertensive episodes have been observed during surgery in patients taking desipramine hydrochloride.
Drug Interactions
DESIPRAMINE HYDROCHLORIDE ADVERSE REACTIONS
Included in the following listing are a few adverse reactions that have not been reported with this specific drug. However, the pharmacologic similarities among the tricyclic antidepressant drugs require that each of the reactions be considered when desipramine hydrochloride is given.
OVERDOSAGE
Deaths may occur from overdosage with this class of drugs. Overdose of desipramine has resulted in a higher death rate compared to overdoses of other tricyclic antidepressants. Multiple drug ingestion (including alcohol) is common in deliberate tricyclic antidepressant overdose. As the management is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity develop rapidly after tricyclic antidepressant overdose; therefore, hospital monitoring is required as soon as possible. There is no specific antidote for desipramine overdosage.
DESIPRAMINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
Not recommended for use in children (see ). WARNINGS
Lower dosages are recommended for elderly patients and adolescents. Lower dosages are also recommended for outpatients compared to hospitalized patients, who are closely supervised. Dosage should be initiated at a low level and increased according to clinical response and any evidence of intolerance. Following remission, maintenance medication may be required for a period of time and should be at the lowest dose that will maintain remission.
MEDICATION GUIDE
Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or Actions
Read the Medication Guide that comes with you or your family member’s antidepressant medicine. This Medication Guide is only about the risk of suicidal thoughts and actions with antidepressant medicines. Talk to your, or your family member’s, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
- Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
- These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions. Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions.
-
How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling very agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
What else do I need to know about antidepressant medicines?
- Stopping an antidepressant medicine suddenly can cause other symptoms. Never stop an antidepressant medicine without first talking to a healthcare provider.
- It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants. Antidepressants are medicines used to treat depression and other illnesses.
- Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member. Antidepressant medicines have other side effects.
- Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider. Antidepressant medicines can interact with other medicines.
- Talk to your child’s healthcare provider for more information. Not all antidepressant medicines prescribed for children are FDA approved for use in children.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
This Medication Guide has been approved by the U.S. Food and Drug Administration for all antidepressants.
Desipramine 25mg Tablet

Desipramine HydrochlorideDesipramine Hydrochloride TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||