Desipramine Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- DESIPRAMINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- DESIPRAMINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- DESIPRAMINE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Suicidality and Antidepressant DrugsAntidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of desipramine hydrochloride or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Desipramine hydrochloride is not approved for use in pediatric patients. (SeeWARNINGS: Clinical Worsening and Suicide Risk,PRECAUTIONS:Information for patientsandPRECAUTIONS:Pediatric use
DESIPRAMINE HYDROCHLORIDE DESCRIPTION
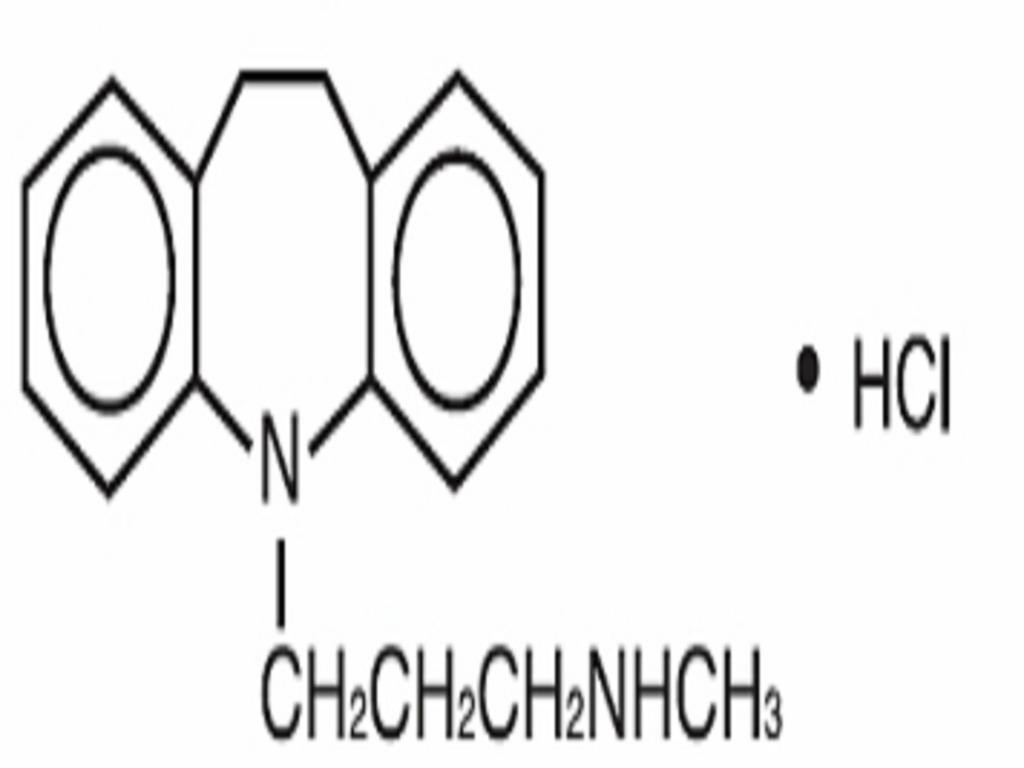
Inactive Ingredients:
CLINICAL PHARMACOLOGY
Mechanism of ActionMetabolism
INDICATIONS & USAGE
DESIPRAMINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Clinical Worsening and Suicide RiskAll patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers.
Screening Patients for Bipolar Disorder
General
Use in Pregnancy
Geriatric Use
DOSAGE AND ADMINISTRATION
ADVERSE REACTIONS
PRECAUTIONS
Information for patients
Clinical Worsening and Suicide Risk
Pediatric use
BOX WARNINGWARNINGS
ADVERSE REACTIONS
General
WARNINGS
DRUG INTERACTIONS
DESIPRAMINE HYDROCHLORIDE ADVERSE REACTIONS
Cardiovascular:
PRECAUTIONSPediatric use
Psychiatric:
Neurologic:
Anticholinergic:
Allergic:
Hematologic:
Gastrointestinal:
Endocrine:
Other:
Withdrawal Symptoms:
DRUG ABUSE AND DEPENDENCE
Overdosage
Oral LD50
Manifestations of Overdosage
ADVERSE REACTIONS
Management
General
Gastrointestinal Decontamination
Cardiovascular
CNS
Psychiatric Follow-up
Pediatric Management
DOSAGE & ADMINISTRATION
WARNINGSUsual Adult Dose
Adolescent and Geriatric Dose
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Desipramine HydrochlorideDesipramine Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!