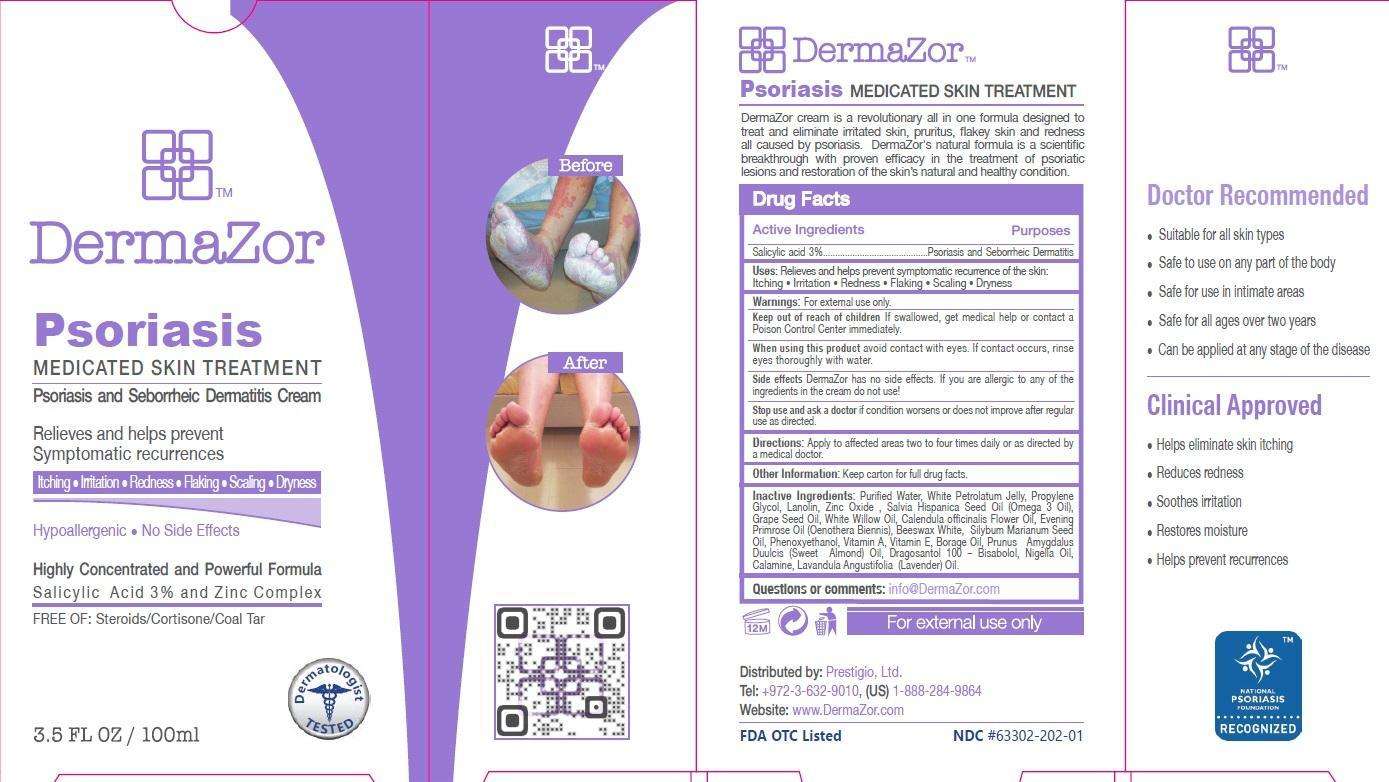
Dermazor
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- Dermazor Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Questions or comments:
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active Ingredients
Salicylic acid 3%
Purpose
Psoriasis and Seborrheic Dermatitis
Dermazor Uses
Relieves and helps prevent symptomatic recurrence of the skin:
- Itching
- Irritation
- Redness
- Flaking
- Scaling
- Dryness
Warnings
For external use only.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center immediately.
When using this product
avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if
condition worsens or does not improve after regular use as directed.
Directions
Apply to the affected areas two to four times daily or as directed by a medical doctor.
Other Information
Keep carton for full drug facts.
Inactive Ingredients
Purified Water, White Petrolatum Jelly, Propylene Glycol, Lanolin, Zinc Oxide, Salvia Hispanica Seed Oil (Omega 3 Oil), Grape Seed Oil, White Willow Oil, Calendula officinalis Flower Oil, Evening Primrose Oil (Oenothera Biennis), Beeswax White, Silybum Marianum Seed Oil, Phenoxyethanol, Vitamin A, Vitamin E, Borage Oil, Prunus Amygdalus Duulcis (Sweet Almond) Oil - Bisabolol, Nigella Oil, Calamine, Lavandula Angustifolia ( Lavender) Oil.
Questions or comments:
info@DermaZor.com
Principal Display Panel
DermaZor
Psoriasis
MEDICATED SKIN TREATMENT
Psoriasis and Seborrheic Dermatitis Cream
Relieves and helps prevent
Symptomatic recurrences
Itching Irritation Redness Flaking Scaling Dryness
Hypoallergenic No side effects
Highly Concentrated and Powerful Formula
Salicylic Acid 3% and Zinc Complex
FREE OF: Steroids/Cortisone/Coal Tar
Dermatologist TESTED
3.5 FL OZ/ 100 ml
DermazorSalicylic Acid, Zinc Oxide CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||