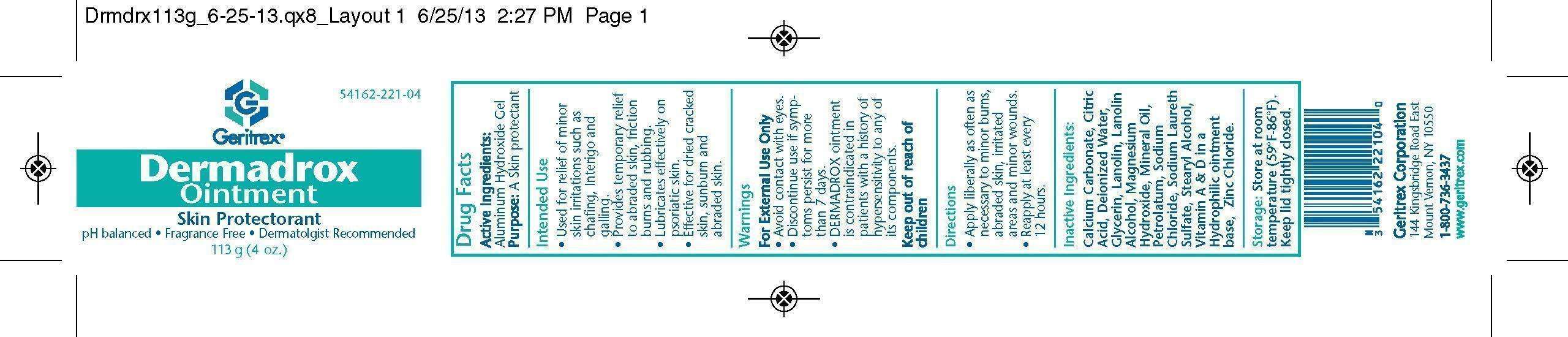
Dermadrox
Dermadrox Ointment
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredients Purpose
Aluminum Hydroxide Gel A skin protectant
Intended Use
Used for relief of minor skin irritations such as chafing, Interigo and galling
Provides temporary relief to abraded skin, friction burns and rubbing
Lubricates effectively on psoriatic skin
Effective for dried cracked skin, sunburn and abraded skin
Warnings
For External Use Only
Avoid contact with eyes
Discontinue use if symptoms persist for more than 7 days
DERMADROX ointment is contraindicated in patients with a history of hypersensitivity to any of its components
Inactive Ingredients
Calcium Carbonate, Citric Acid, Deionized Water, Glycerin, Lanolin, Lanolin Alcohol, Magnesium Hydroxide, Mineral Oil,
Petrolatum, Sodium Chloride, Sodium Laureth Sulfate, Stearyl Alcohol, Vitamin A and D, Zinc Chloride
Keep out of reach of children
Storage
Store at room temperature (59'F-86'F) Keep lid tightly closed
Reapply at least every 12 hours
Direction
Apply liberally as often as necessary to minor burns, abraded skin, irritated areas and minor wounds
Reapply at least every 12 hours
DermadroxAluminum Hydroxide OINTMENT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||