Home – Degree UltraClear Pure Satin
Degree UltraClear Pure Satin
Conopco Inc. d/b/a Unilever
PDP and drug facts for Degree UltraClear Pure Satin AP and Deodorant
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient PurposeWarningsFor external use onlyDo not useAsk a doctor before use if you haveStop useKeep out of reach of children.Questions?1-866 DEGREE1
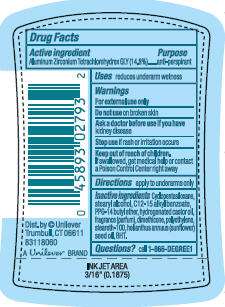
Degree UltraClear Pure Satin
Aluminum Zirconium Tetrachlorohydrex GLY STICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:64942-0779 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:64942-0779-1 |
74 in 1 CONTAINER |
|
|
|
2 |
NDC:64942-0779-2 |
45 in 1 CONTAINER |
|
|
|
3 |
NDC:64942-0779-3 |
14 in 1 CONTAINER |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part350 |
2009-04-28 |
|
|
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!

