Dallergy
Laser Pharmaceuticals, LLC
Woodfield Pharmaceutical, LLC
Dallergy SYRUP Improved Formulation
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Active ingredients
(in each 5 mL teaspoonful)
Dexbrompheniramine Maleate 1 mg
Phenylephrine Hydrochloride 5 mg
Purpose
Purpose
Antihistamine
Nasal Decongestant
Uses
Uses temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
- nasal congestion
- reduces swelling of nasal passages
Warnings
Do not exceed recommended dosage.Do not use this product
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- heart disease
- high blood pressure
- thyroid disease
- diabetes
Ask a doctor or pharmacist before use if you ar e taking sedatives or tranquilizers.
When using this product
- excitability may occur, especially in children
- may cause drowsiness
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase the drowsiness effect
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- symptoms do not improve within 7 days or are accompanied by fever
- new symptoms occur
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
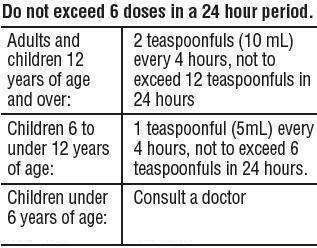
Directions
Other information
Store at 59°-86°F (15°-30°C)
Inactive ingredients
Citric Acid, FD&C Blue #1, FD&C Red #40, Glycerin, Grape Flavor, Propylene Glycol, Purified Water, Sodium Citrate, Sodium Saccharin, Sorbitol.
Questions? Comments?
Call 1-864-286-8229
LASER
Manufactured for:
Laser Pharmaceuticals, LLC
Greenville, SC 29615
Product Packaging
The packaging below represents the labeling currently used.
Principal display panel and side panel for 473 mL label:
NDC: 16477-822-01
Dallergy®
SYRUP
Improved
Formulation
Antihistamine
Nasal Decongestant
Each 5 mL (1 teaspoonful) contains:
Dexbrompheniramine Maleate.............1
mg
Phenylephrine HCl................................5 mg
Grape Flavor
LASER
16 fl oz (473 mL)
For full prescribing information, see the product foldout.
Store at 59°-86°F (15°-30°C)
Tamper-evident by foil seal under cap. Do not use if foil seal is
broken or missing.
Dispense in a tight, light-resistant container as defined in the
USP/NF with a child-resistant closure.
WARNING: KEEP THIS AND ALL DRUGS OUT OF REACH OF
CHILDREN. IN CASE OF OVERDOSE, GET MEDICAL HELP OR
CONTACT A POISON CONTROL CENTER RIGHT AWAY.
LASER
Manufactured for:
Laser Pharmaceuticals, LLC
Greenville, SC 29615
Patent Pending
Rev. 11/13


DallergyDexbrompheniramine Maleate, Phenylephrine Hydrochloride SYRUP
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||