Dacogen
HIGHLIGHTS OF PRESCRIBING INFORMATION INDICATIONS AND USAGEDacogen is a nucleoside metabolic inhibitor indicated for treatment of patients with myelodysplastic syndromes (MDS) including previously treated and untreated, de novo and secondary MDS of all French-American-British subtypes (refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, and chronic myelomonocytic leukemia) and intermediate-1, intermediate-2, and high-risk International Prognostic Scoring System groups. (1)DOSAGE AND ADMINISTRATIONThere are two regimens for Dacogen administration. With either regimen it is recommended that patients be treated for a minimum of 4 cycles; however, a complete or partial response may take longer than 4 cycles. (2) Treatment Regimen – Option 1 Administer Dacogen at a dose of 15 mg/m2 by continuous intravenous infusion over 3 hours repeated every 8 hours for 3 days. Repeat cycle every 6 weeks. (2.1) Treatment Regimen – Option 2 Administer Dacogen at a dose of 20 mg/m2 by continuous intravenous infusion over 1 hour repeated daily for 5 days. Repeat cycle every 4 weeks. (2.2) DOSAGE FORMS AND STRENGTHSLyophilized powder in a single-dose vial, 50 mg/vial. (3) CONTRAINDICATIONSNone WARNINGS AND PRECAUTIONS Neutropenia and thrombocytopenia: Perform complete blood counts and platelet counts. (5.1) Pregnancy: Can cause fetal harm. Advise women of potential risk to the fetus (5.2, 8.1) Women of childbearing potential and men with female partners of childbearing potential should use effective contraception and avoid pregnancy (5.3, 5.4) Side EffectsMost common adverse reactions (> 50%) are neutropenia, thrombocytopenia, anemia, and pyrexia. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Eisai, Inc. at 1-888-274-2378 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 DACOGEN INDICATIONS AND USAGE
- 2 DACOGEN DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 DACOGEN CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 DACOGEN ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 DACOGEN DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Dacogen is indicated for treatment of patients with myelodysplastic syndromes (MDS) including previously treated and untreated, de novo and secondary MDS of all French-American-British subtypes (refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, and chronic myelomonocytic leukemia) and intermediate-1, intermediate-2, and high-risk International Prognostic Scoring System groups.
2 DOSAGE AND ADMINISTRATION
There are two regimens for Dacogen administration. With either regimen it is recommended that patients be treated for a minimum of 4 cycles; however, a complete or partial response may take longer than 4 cycles.
Complete blood counts and platelet counts should be performed as needed to monitor response and toxicity, but at a minimum, prior to each cycle. Liver chemistries and serum creatinine should be obtained prior to initiation of treatment.
2.1 Treatment Regimen – Option 1
Dacogen is administered at a dose of 15 mg/m2 by continuous intravenous infusion over 3 hours repeated every 8 hours for 3 days. This cycle should be repeated every 6 weeks. Patients may be premedicated with standard anti-emetic therapy.
If hematologic recovery (ANC ≥ 1,000/μL and platelets ≥ 50,000/μL) from a previous Dacogen treatment cycle requires more than 6 weeks, then the next cycle of Dacogen therapy should be delayed and dosing temporarily reduced by following this algorithm:
- Recovery requiring more than 6, but less than 8 weeks − Dacogen dosing to be delayed for up to 2 weeks and the dose temporarily reduced to 11 mg/m2 every 8 hours (33 mg/m2/day, 99 mg/m2/cycle) upon restarting therapy.
- Recovery requiring more than 8, but less than 10 weeks − Patient should be assessed for disease progression (by bone marrow aspirates); in the absence of progression, the Dacogen dose should be delayed up to 2 more weeks and the dose reduced to 11 mg/m2 every 8 hours (33 mg/m2/day, 99 mg/m2/cycle) upon restarting therapy, then maintained or increased in subsequent cycles as clinically indicated.
2.2 Treatment Regimen – Option 2
Dacogen is administered at a dose of 20 mg/m2 by continuous intravenous infusion over 1 hour repeated daily for 5 days. This cycle should be repeated every 4 weeks. Patients may be premedicated with standard anti-emetic therapy.
If myelosuppression is present, subsequent treatment cycles of Dacogen should be delayed until there is hematologic recovery (ANC ≥ 1,000/μL platelets ≥ 50,000/μL ).
2.3 Patients with Non-hematologic Toxicity
Following the first cycle of Dacogen treatment, if any of the following non-hematologic toxicities are present, Dacogen treatment should not be restarted until the toxicity is resolved: 1) serum creatinine ≥ 2 mg/dL; 2) SGPT, total bilirubin ≥ 2 times ULN; 3) and active or uncontrolled infection.
2.4 Instructions for Intravenous Administration
Dacogen is a cytotoxic drug and caution should be exercised when handling and preparing Dacogen. Procedures for proper handling and disposal of antineoplastic drugs should be applied. Several guidances on this subject have been published.1-4.
Dacogen should be aseptically reconstituted with 10 mL of Sterile Water for Injection (USP); upon reconstitution, each mL contains approximately 5.0 mg of decitabine at pH 6.7-7.3. Immediately after reconstitution, the solution should be further diluted with 0.9% Sodium Chloride Injection or 5% Dextrose Injection to a final drug concentration of 0.1 - 1.0 mg/mL. Unless used within 15 minutes of reconstitution, the diluted solution must be prepared using cold (2˚C - 8˚C) infusion fluids and stored at 2˚C - 8˚C (36˚F - 46˚F) for up to a maximum of 4 hours until administration.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if there is evidence of particulate matter or discoloration.
3 DOSAGE FORMS AND STRENGTHS
Dacogen (decitabine) for Injection is supplied as a sterile, lyophilized white to almost white powder, in a single-dose vial, packaged in cartons of 1 vial. Each vial contains 50 mg of decitabine.
4 CONTRAINDICATIONS
None
5 WARNINGS AND PRECAUTIONS
5.1 Neutropenia and Thrombocytopenia
Treatment with Dacogen is associated with neutropenia and thrombocytopenia. Complete blood and platelet counts should be performed as needed to monitor response and toxicity, but at a minimum, prior to each dosing cycle. After administration of the recommended dosage for the first cycle, treatment for subsequent cycles should be adjusted [see Dosage and Administration (2.1, 2.2)]. Clinicians should consider the need for early institution of growth factors and/or antimicrobial agents for the prevention or treatment of infections in patients with MDS. Myelosuppression and worsening neutropenia may occur more frequently in the first or second treatment cycles, and may not necessarily indicate progression of underlying MDS.
5.2 Use in Pregnancy
Dacogen can cause fetal harm when administered to a pregnant woman. Based on its mechanism of action, Dacogen is expected to result in adverse reproductive effects. In preclinical studies in mice and rats, decitabine was teratogenic, fetotoxic, and embryotoxic. There are no adequate and well-controlled studies of Dacogen in pregnant women. If this drug is used during pregnancy, or if a patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant while taking Dacogen [see Use in Specific Populations (8.1)]
5.3 Use in Women of Childbearing Potential
Women of childbearing potential should be advised to avoid becoming pregnant while receiving Dacogen and for 1 month following completion of treatment. Women of childbearing potential should be counseled to use effective contraception during this time [see Use in Specific Populations (8.1)]. Based on its mechanism of action, Dacogen can cause fetal harm if used during pregnancy.
5.4 Use in Men
Men should be advised not to father a child while receiving treatment with Dacogen, and for 2 months following completion of treatment [see Nonclinical Toxicology (13.1)]. Men with female partners of childbearing potential should use effective contraception during this time. Based on its mechanism of action, Dacogen alters DNA synthesis and can cause fetal harm.
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Most Commonly Occurring Adverse Reactions: neutropenia, thrombocytopenia, anemia, fatigue, pyrexia, nausea, cough, petechiae, constipation, diarrhea, and hyperglycemia.
Adverse Reactions Most Frequently (≥ 1%) Resulting in Clinical Intervention in the Phase 3 Trials in the Dacogen Arm:
- Discontinuation: thrombocytopenia, neutropenia, pneumonia, Mycobacterium avium complex infection, cardio-respiratory arrest, increased blood bilirubin, intracranial hemorrhage, abnormal liver function tests.
- Dose Delayed: neutropenia, pulmonary edema, atrial fibrillation, central line infection, febrile neutropenia.
- Dose Reduced: neutropenia, thrombocytopenia, anemia, lethargy, edema, tachycardia, depression, pharyngitis.
Discussion of Adverse Reactions Information
Dacogen was studied in 3 single-arm studies (N = 66, N = 98, N = 99) and 1 controlled supportive care study (N = 83 Dacogen, N = 81 supportive care ). The data described below reflect exposure to Dacogen in 83 patients in the MDS trial. In the trial, patients received 15 mg/m2 intravenously every 8 hours for 3 days every 6 weeks. The median number of Dacogen cycles was 3 (range 0 to 9).
Table 1 presents all adverse events regardless of causality occurring in at least 5% of patients in the Dacogen group and at a rate greater than supportive care.
| Dacogen N = 83 (%) |
Supportive Care N = 81 (%) |
|
|---|---|---|
| Blood and lymphatic system disorders | ||
| Neutropenia | 75 (90) | 58 (72) |
| Thrombocytopenia | 74 (89) | 64 (79) |
| Anemia NOS | 68 (82) | 60 (74) |
| Febrile neutropenia | 24 (29) | 5 (6) |
| Leukopenia NOS | 23 (28) | 11 (14) |
| Lymphadenopathy | 10 (12) | 6 (7) |
| Thrombocythemia | 4 (5) | 1 (1) |
| Cardiac disorders | ||
| Pulmonary edema NOS | 5 (6) | 0 (0) |
| Eye disorders | ||
| Vision blurred | 5 (6) | 0 (0) |
| Gastrointestinal disorders | ||
| Nausea | 35 (42) | 13 (16) |
| Constipation | 29 (35) | 11 (14) |
| Diarrhea NOS | 28 (34) | 13 (16) |
| Vomiting NOS | 21 (25) | 7 (9) |
| Abdominal pain NOS | 12 (14) | 5 (6) |
| Oral mucosal petechiae | 11 (13) | 4 (5) |
| Stomatitis | 10 (12) | 5 (6) |
| Dyspepsia | 10 (12) | 1 (1) |
| Ascites | 8 (10) | 2 (2) |
| Gingival bleeding | 7 (8) | 5 (6) |
| Hemorrhoids | 7 (8) | 3 (4) |
| Loose stools | 6 (7) | 3 (4) |
| Tongue ulceration | 6 (7) | 2 (2) |
| Dysphagia | 5 (6) | 2 (2) |
| Oral soft tissue disorder NOS | 5 (6) | 1 (1) |
| Lip ulceration | 4 (5) | 3 (4) |
| Abdominal distension | 4 (5) | 1 (1) |
| Abdominal pain upper | 4 (5) | 1 (1) |
| Gastro-esophageal reflux disease | 4 (5) | 0 (0) |
| Glossodynia | 4 (5) | 0 (0) |
| General disorders and administrative site disorders | ||
| Pyrexia | 44 (53) | 23 (28) |
| Edema peripheral | 21 (25) | 13 (16) |
| Rigors | 18 (22) | 14 (17) |
| Edema NOS | 15 (18) | 5 (6) |
| Pain NOS | 11 (13) | 5 (6) |
| Lethargy | 10 (12) | 3 (4) |
| Tenderness NOS | 9 (11) | 0 (0) |
| Fall | 7 (8) | 3 (4) |
| Chest discomfort | 6 (7) | 3 (4) |
| Intermittent pyrexia | 5 (6) | 3 (4) |
| Malaise | 4 (5) | 1 (1) |
| Crepitations NOS | 4 (5) | 1 (1) |
| Catheter site erythema | 4 (5) | 1 (1) |
| Catheter site pain | 4 (5) | 0 (0) |
| Injection site swelling | 4 (5) | 0 (0) |
| Hepatobiliary disorders | ||
| Hyperbilirubinemia | 12 (14) | 4 (5) |
| Infections and infestations | ||
| Pneumonia NOS | 18 (22) | 11 (14) |
| Cellulitis | 10 (12) | 6 (7) |
| Candidal infection NOS | 8 (10) | 1 (1) |
| Catheter related infection | 7 (8) | 0 (0) |
| Urinary tract infection NOS | 6 (7) | 1 (1) |
| Staphylococcal infection | 6 (7) | 0 (0) |
| Oral candidiasis | 5 (6) | 2 (2) |
| Sinusitis NOS | 4 (5) | 2 (2) |
| Bacteremia | 4 (5) | 0 (0) |
| Injury, poisoning and procedural complications | ||
| Transfusion reaction | 6 (7) | 3 (4) |
| Abrasion NOS | 4 (5) | 1 (1) |
| Investigations | ||
| Cardiac murmur NOS | 13 (16) | 9 (11) |
| Blood alkaline phosphatase NOS increased | 9 (11) | 7 (9) |
| Aspartate aminotransferase increased | 8 (10) | 7 (9) |
| Blood urea increased | 8 (10) | 1 (1) |
| Blood lactate dehydrogenase increased | 7 (8) | 5 (6) |
| Blood albumin decreased | 6 (7) | 0 (0) |
| Blood bicarbonate increased | 5 (6) | 1 (1) |
| Blood chloride decreased | 5 (6) | 1 (1) |
| Protein total decreased | 4 (5) | 3 (4) |
| Blood bicarbonate decreased | 4 (5) | 1 (1) |
| Blood bilirubin decreased | 4 (5) | 1 (1) |
| Metabolism and nutrition disorders | ||
| Hyperglycemia NOS | 27 (33) | 16 (20) |
| Hypoalbuminemia | 20 (24) | 14 (17) |
| Hypomagnesemia | 20 (24) | 6 (7) |
| Hypokalemia | 18 (22) | 10 (12) |
| Hyponatremia | 16 (19) | 13 (16) |
| Appetite decreased NOS | 13 (16) | 12 (15) |
| Anorexia | 13 (16) | 8 (10) |
| Hyperkalemia | 11 (13) | 3 (4) |
| Dehydration | 5 (6) | 4 (5) |
| Musculoskeletal and connective tissue disorders | ||
| Arthralgia | 17 (20) | 8 (10) |
| Pain in limb | 16 (19) | 8 (10) |
| Back pain | 14 (17) | 5 (6) |
| Chest wall pain | 6 (7) | 1 (1) |
| Musculoskeletal discomfort | 5 (6) | 0 (0) |
| Myalgia | 4 (5) | 1 (1) |
| Nervous system disorders | ||
| Headache | 23 (28) | 11 (14) |
| Dizziness | 15 (18) | 10 (12) |
| Hypoesthesia | 9 (11) | 1 (1) |
| Psychiatric disorders | ||
| Insomnia | 23 (28) | 11 (14) |
| Confusional state | 10 (12) | 3 (4) |
| Anxiety | 9 (11) | 8 (10) |
| Renal and urinary disorders | ||
| Dysuria | 5 (6) | 3 (4) |
| Urinary frequency | 4 (5) | 1 (1) |
| Respiratory, thoracic and Mediastinal disorders | ||
| Cough | 33 (40) | 25 (31) |
| Pharyngitis | 13 (16) | 6 (7) |
| Crackles lung | 12 (14) | 1 (1) |
| Breath sounds decreased | 8 (10) | 7 (9) |
| Hypoxia | 8 (10) | 4 (5) |
| Rales | 7 (8) | 2 (2) |
| Postnasal drip | 4 (5) | 2 (2) |
| Skin and subcutaneous tissue disorders | ||
| Ecchymosis | 18 (22) | 12 (15) |
| Rash NOS | 16 (19) | 7 (9) |
| Erythema | 12 (14) | 5 (6) |
| Skin lesion NOS | 9 (11) | 3 (4) |
| Pruritis | 9 (11) | 2 (2) |
| Alopecia | 7 (8) | 1 (1) |
| Urticaria NOS | 5 (6) | 1 (1) |
| Swelling face | 5 (6) | 0 (0) |
| Vascular disorders | ||
| Petechiae | 32 (39) | 13 (16) |
| Pallor | 19 (23) | 10 (12) |
| Hypotension NOS | 5 (6) | 4 (5) |
| Hematoma NOS | 4 (5) | 3 (4) |
Discussion of Clinically Important Adverse Reactions
In the controlled trial using Dacogen dosed at 15 mg/m2, administered by continuous intravenous infusion over 3 hours repeated every 8 hours for 3 days, the highest incidence of Grade 3 or Grade 4 adverse events in the Dacogen arm were neutropenia (87%), thrombocytopenia (85%), febrile neutropenia (23%) and leukopenia (22%). Bone marrow suppression was the most frequent cause of dose reduction, delay and discontinuation. Six patients had fatal events associated with their underlying disease and myelosuppression (anemia, neutropenia, and thrombocytopenia) that were considered at least possibly related to drug treatment [See Warnings and Precautions (5.1)]. Of the 83 Dacogen-treated patients, 8 permanently discontinued therapy for adverse events; compared to 1 of 81 patients in the supportive care arm.
In a single-arm MDS study (N=99) Dacogen was dosed at 20 mg/m2 intravenous, infused over one hour daily for 5 consecutive days of a 4 week cycle. Table 2 presents all adverse events regardless of causality occurring in at least 5% of patients.
| Dacogen N = 99 (%) |
|
|---|---|
| Blood and lymphatic system disorders | |
| Anemia | 31 (31%) |
| Febrile neutropenia | 20 (20%) |
| Leukopenia | 6 (6% ) |
| Neutropenia | 38 (38% ) |
| Pancytopenia | 5 (5% ) |
| Thrombocythemia | 5 (5% ) |
| Thrombocytopenia | 27 (27%) |
| Cardiac disorders | |
| Cardiac failure congestive | 5 (5% ) |
| Tachycardia | 8 (8% ) |
| Ear and labyrinth disorders | |
| Ear pain | 6 (6% ) |
| Gastrointestinal disorders | |
| Abdominal pain | 14 (14%) |
| Abdominal pain upper | 6 (6% ) |
| Constipation | 30 (30%) |
| Diarrhea | 28 (28%) |
| Dyspepsia | 10 (10%) |
| Dysphagia | 5 (5% ) |
| Gastro-esophageal reflux disease | 5 (5% ) |
| Nausea | 40 (40%) |
| Oral pain | 5 (5% ) |
| Stomatitis | 11 (11%) |
| Toothache | 6 (6% ) |
| Vomiting | 16 (16%) |
| General disorders and administration site conditions | |
| Asthenia | 15 (15%) |
| Chest pain | 6 (6% ) |
| Chills | 16 (16%) |
| Fatigue | 46 (46%) |
| Mucosal inflammation | 9 (9% ) |
| Edema | 5 (5% ) |
| Edema peripheral | 27 (27%) |
| Pain | 5 (5% ) |
| Pyrexia | 36 (36%) |
| Infections and infestations | |
| Cellulitis | 9 (9% ) |
| Oral candidiasis | 6 (6% ) |
| Pneumonia | 20 (20%) |
| Sinusitis | 6 (6% ) |
| Staphylococcal bacteremia | 8 (8% ) |
| Tooth abscess | 5 (5% ) |
| Upper respiratory tract infection | 10 (10%) |
| Urinary tract infection | 7 (7% ) |
| Injury, poisoning and procedural complications | |
| Contusion | 9 (9% ) |
| Investigations | |
| Blood bilirubin increased | 6 (6% ) |
| Breath sounds abnormal | 5 (5% ) |
| Weight decreased | 9 (9% ) |
| Metabolism and nutrition disorders | |
| Anorexia | 23 (23%) |
| Decreased appetite | 8 (8% ) |
| Dehydration | 8 (8% ) |
| Hyperglycemia | 6 (6% ) |
| Hypokalemia | 12 (12%) |
| Hypomagnesemia | 5 (5% ) |
| Musculoskeletal and connective tissue disorders | |
| Arthralgia | 17 (17%) |
| Back pain | 18 (18%) |
| Bone pain | 6 (6% ) |
| Muscle spasms | 7 (7% ) |
| Muscular weakness | 5 (5% ) |
| Musculoskeletal pain | 5 (5% ) |
| Myalgia | 9 (9% ) |
| Pain in extremity | 18 (18%) |
| Nervous system disorders | |
| Dizziness | 21 (21%) |
| Headache | 23 (23%) |
| Psychiatric disorders | |
| Anxiety | 9 (9% ) |
| Confusional state | 8 (8% ) |
| Depression | 9 (9% ) |
| Insomnia | 14 (14%) |
| Respiratory, thoracic and mediastinal disorders | |
| Cough | 27 (27%) |
| Dyspnea | 29 (29%) |
| Epistaxis | 13 (13%) |
| Pharyngolaryngeal pain | 8 (8% ) |
| Pleural effusion | 5 (5% ) |
| Sinus congestion | 5 (5% ) |
| Skin and subcutaneous tissue disorders | |
| Dry skin | 8 (8% ) |
| Ecchymosis | 9 (9% ) |
| Erythema | 5 (5% ) |
| Night sweats | 5 (5% ) |
| Petechiae | 12 (12%) |
| Pruritus | 9 (9% ) |
| Rash | 11 (11%) |
| Skin lesion | 5 (5% ) |
| Vascular disorders | |
| Hypertension | 6 (6% ) |
| Hypotension | 11 (11%) |
| * In this single arm study, investigators reported adverse events based on clinical signs and symptoms rather than predefined laboratory abnormalities. Thus not all laboratory abnormalities were recorded as adverse events. |
|
Discussion of Clinically Important Adverse Reactions
In the single-arm study (N=99) when Dacogen was dosed at 20 mg/m2 intravenous, infused over one hour daily for 5 consecutive days, the highest incidence of Grade 3 or Grade 4 adverse events were neutropenia (37%), thrombocytopenia (24%) and anemia (22%). Seventy-eight percent of patients had dose delays, the median duration of this delay was 7 days and the largest percentage of delays were due to hematologic toxicities. Hematologic toxicities and infections were the most frequent causes of dose delays and discontinuation. Eight patients had fatal events due to infection and/or bleeding (seven of which occurred in the clinical setting of myelosuppression) that were considered at least possibly related to drug treatment. Nineteen of 99 patients permanently discontinued therapy for adverse events.
No overall difference in safety was detected between patients > 65 years of age and younger patients in these myelodysplasia trials. No significant gender differences in safety or efficacy were detected. Patients with renal or hepatic dysfunction were not studied. Insufficient numbers of non-white patients were available to draw conclusions in these clinical trials.
Serious Adverse Events that occurred in patients receiving Dacogen regardless of causality, not previously reported in Tables 1 and 2 include:
- Blood and Lymphatic System Disorders: myelosuppression, splenomegaly.
- Cardiac Disorders: myocardial infarction, cardio-respiratory arrest, cardiomyopathy, atrial fibrillation, supraventricular tachycardia.
- Gastrointestinal Disorders: gingival pain, upper gastrointestinal hemorrhage.
- General Disorders and Administrative Site Conditions: chest pain, catheter site hemorrhage.
- Hepatobiliary Disorders: cholecystitis.
- Infections and Infestations: fungal infection, sepsis, bronchopulmonary aspergillosis, peridiverticular abscess, respiratory tract infection, pseudomonal lung infection, Mycobacterium avium complex infection.
- Injury, Poisoning and Procedural Complications: post procedural pain, post procedural hemorrhage.
- Nervous System Disorders: intracranial hemorrhage.
- Psychiatric Disorders: mental status changes.
- Renal and Urinary Disorders: renal failure, urethral hemorrhage.
- Respiratory, Thoracic and Mediastinal Disorders: hemoptysis, lung infiltration, pulmonary embolism, respiratory arrest, pulmonary mass.
- Allergic Reaction: Hypersensitivity (anaphylactic reaction) to Dacogen has been reported in a Phase 2 trial.
6.2 Post-marketing Experience
The following adverse reactions have been identified during post-approval use of Dacogen. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cases of Sweet’s Syndrome (acute febrile neutrophilic dermatosis) have been reported.
7 DRUG INTERACTIONS
Drug interaction studies with decitabine have not been conducted. In vitro studies in human liver microsomes suggest that decitabine is unlikely to inhibit or induce cytochrome P450 enzymes. In vitro metabolism studies have suggested that decitabine is not a substrate for human liver cytochrome P450 enzymes. As plasma protein binding of decitabine is negligible (<1%), interactions due to displacement of more highly protein bound drugs from plasma proteins are not expected.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Dacogen can cause fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies of Dacogen in pregnant women.
The developmental toxicity of decitabine was examined in mice exposed to single IP (intraperitoneal) injections (0, 0.9 and 3.0 mg/m2, approximately 2% and 7% of the recommended daily clinical dose, respectively) over gestation days 8, 9, 10 or 11. No maternal toxicity was observed but reduced fetal survival was observed after treatment at 3 mg/m2 and decreased fetal weight was observed at both dose levels. The 3 mg/m2 dose elicited characteristic fetal defects for each treatment day, including supernumerary ribs (both dose levels), fused vertebrae and ribs, cleft palate, vertebral defects, hind-limb defects and digital defects of fore- and hind-limbs. In rats given a single IP injection of 2.4, 3.6 or 6 mg/m2 (approximately 5, 8, or 13% the daily recommended clinical dose, respectively) on gestation days 9-12, no maternal toxicity was observed. No live fetuses were seen at any dose when decitabine was injected on gestation day 9. A significant decrease in fetal survival and reduced fetal weight at doses greater than 3.6 mg/m2 was seen when decitabine was given on gestation day 10. Increased incidences of vertebral and rib anomalies were seen at all dose levels, and induction of exophthalmia, exencephaly, and cleft palate were observed at 6.0 mg/m2. Increased incidence of foredigit defects was seen in fetuses at doses greater than 3.6 mg/m2. Reduced size and ossification of long bones of the fore-limb and hind-limb were noted at 6.0 mg/m2. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of child bearing potential should be advised to avoid becoming pregnant while taking Dacogen.
8.3 Nursing Mothers
It is not known whether decitabine or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions from Dacogen in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and effectiveness of Dacogen in pediatric patients have not been established.
8.5 Geriatric Use
Of the total number of patients exposed to Dacogen in the controlled clinical trial, 61 of 83 patients were age 65 and over, while 21 of 83 patients were age 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
There are no data on the use of Dacogen in patients with renal dysfunction; therefore, Dacogen should be used with caution in these patients.
8.7 Hepatic Impairment
There are no data on the use of Dacogen in patients with hepatic dysfunction; therefore, Dacogen should be used with caution in these patients.
10 OVERDOSAGE
There is no known antidote for overdosage with Dacogen. Higher doses are associated with increased myelosuppression including prolonged neutropenia and thrombocytopenia. Standard supportive measures should be taken in the event of an overdose.
11 DESCRIPTION
Dacogen (decitabine) for Injection contains decitabine (5-aza-2’-deoxycitidine), an analogue of the natural nucleoside 2’-deoxycytidine. Decitabine is a fine, white to almost white powder with the molecular formula of C8H12N4O4 and a molecular weight of 228.21. Its chemical name is 4-amino-1-(2-deoxy-β-D-erythro-pentofuranosyl)-1,3,5-triazin-2(1H)-one and it has the following structural formula:
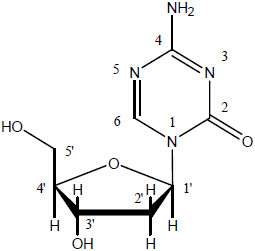
Decitabine is slightly soluble in ethanol/water (50/50), methanol/water (50/50) and methanol; sparingly soluble in water and soluble in dimethylsulfoxide (DMSO).
Dacogen (decitabine) for Injection is a white to almost white sterile lyophilized powder supplied in a clear colorless glass vial. Each 20 mL, single dose, glass vial contains 50 mg decitabine, 68 mg monobasic potassium phosphate (potassium dihydrogen phosphate) and 11.6 mg sodium hydroxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Decitabine is believed to exert its antineoplastic effects after phosphorylation and direct incorporation into DNA and inhibition of DNA methyltransferase, causing hypomethylation of DNA and cellular differentiation or apoptosis. Decitabine inhibits DNA methylation in vitro, which is achieved at concentrations that do not cause major suppression of DNA synthesis. Decitabine-induced hypomethylation in neoplastic cells may restore normal function to genes that are critical forthe control of cellulardifferentiation and proliferation. In rapidly dividing cells, the cytotoxicity of decitabine may also be attributed to the formation of covalent adducts between DNA methyltransferase and decitabine incorporated into DNA. Non-proliferating cells are relatively insensitive to decitabine.
12.2 Pharmacodynamics
Decitabine has been shown to induce hypomethylation both in vitro and in vivo. However, there have been no studies of decitabine-induced hypomethylation and pharmacokinetic parameters.
12.3 Pharmacokinetics
Pharmacokinetic parameters were evaluated in patients. Eleven patients received 20 mg/m2 infused over 1 hour intravenously (treatment Option 2), Fourteen patients received 15 mg/m2 infused over 3 hours (treatment Option 1). PK parameters are shown in Table 3. Plasma concentration-time profiles after discontinuation of infusion showed a biexponential decline. The CL of decitabine was higher following treatment Option 2. Upon repeat doses there was no systemic accumulation of decitabine or any changes in PK parameters. Population PK analysis (N=35) showed that the cumulative AUC per cycle for treatment Option 2 was 2.3-fold lower than the cumulative AUC per cycle following treatment Option 1.
| Dose | Cmax
(ng/mL) |
AUC0-∞
(ng·h/mL) |
T1/2
(h) |
CL (L/h/m2) |
AUCCumulative*** (ng·h/mL) |
|---|---|---|---|---|---|
| *N=14, **N=11, ***N=35 Cumulative AUC per cycle | |||||
| 15 mg/m2 3-hr infusion every 8 hours for 3 days (Option 1)* | 73.8 (66) |
163 (62) |
0.62 (49) |
125 (53) |
1332 (1010-1730) |
| 20 mg/m2 1-hr infusion daily for 5 days (Option 2)** | 147 (49) |
115 (43) |
0.54 (43) |
210 (47) |
570 (470-700) |
The exact route of elimination and metabolic fate of decitabine is not known in humans. One of the pathways of elimination of decitabine appears to be deamination by cytidine deaminase found principally in the liver but also in granulocytes, intestinal epithelium and whole blood.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
Carcinogenicity studies with decitabine have not been conducted.
The mutagenic potential of decitabine was tested in several in vitro and in vivo systems. Decitabine increased mutation frequency in L5178Y mouse lymphoma cells, and mutations were produced in an Escherichia coli lac-I transgene in colonic DNA of decitabine-treated mice. Decitabine caused chromosomal rearrangements in larvae of fruit flies.
The effect of decitabine on postnatal development and reproductive capacity was evaluated in mice administered a single 3 mg/m2 IP injection (approximately 7% the recommended daily clinical dose) on day 10 of gestation. Body weights of males and females exposed in utero to decitabine were significantly reduced relative to controls at all postnatal time points. No consistent effect on fertility was seen when female mice exposed in utero were mated to untreated males. Untreated females mated to males exposed in utero showed decreased fertility at 3 and 5 months of age (36% and 0% pregnancy rate, respectively). In male mice given IP injections of 0.15, 0.3 or 0.45 mg/m2 decitabine (approximately 0.3% to 1% the recommended clinical dose) 3 times a week for 7 weeks, decitabine did not affect survival, body weight gain or hematological measures (hemoglobin and WBC counts). Testes weights were reduced, abnormal histology was observed and significant decreases in sperm number were found at doses ≥ 0.3 mg/m2. In females mated to males dosed with ≥ 0.3 mg/m2 decitabine, pregnancy rate was reduced and preimplantation loss was significantly increased.
14 CLINICAL STUDIES
14.1 Controlled Trial
A randomized open-label, multicenter, controlled trial evaluated 170 adult patients with myelodysplastic syndromes (MDS) meeting French-American-British (FAB) classification criteria and International Prognostic Scoring System (IPSS) High-Risk, Intermediate-2 and Intermediate-1 prognostic scores. Eighty-nine patients were randomized to Dacogen therapy plus supportive care (only 83 received Dacogen), and 81 to Supportive Care (SC) alone. Patients with Acute Myeloid Leukemia (AML) were not intended to be included. Of the 170 patients included in the study, independent review (adjudicated diagnosis) found that 12 patients (9 in the Dacogen arm and 3 in the SC arm) had the diagnosis of AML at baseline. Baseline demographics and other patient characteristics in the Intent-to-Treat (ITT) population were similar between the 2 groups, as shown in Table 4.
| Demographic or Other Patient Characteristic | Dacogen N = 89 |
Supportive Care N = 81 |
|---|---|---|
| Age (years) | ||
| Mean (±SD) Median (IQR) (Range: min-max) |
69±10 70 (65-76) (31-85) |
67±10 70 (62-74) (30-82) |
| Gender n (%) | ||
| Male Female |
59 (66) 30 (34) |
57 (70) 24 (30) |
| Race n (%) | ||
| White Black Other |
83 (93) 4 (4) 2 (2) |
76 (94) 2 (2) 3 (4) |
| Weeks Since MDS Diagnosis | ||
| Mean (±SD) Median (IQR) (Range: min-max) |
86±131 29 (10-87) (2-667) |
77±119 35 (7-98) (2-865) |
| Previous MDS Therapy n (%) | ||
| Yes No |
27 (30) 62 (70) |
19 (23) 62 (77) |
| RBC Transfusion Status n (%) | ||
| Independent Dependent |
23 (26) 66 (74) |
27 (33) 54 (67) |
| Platelet Transfusion Status n (%) | ||
| Independent Dependent |
69 (78) 20 (22) |
62 (77) 19 (23) |
| IPSS Classification n (%) | ||
| Intermediate-1 Intermediate-2 High Risk |
28 (31) 38 (43) 23 (26) |
24 (30) 36 (44) 21 (26) |
| FAB Classification n (%) | ||
| RA RARS RAEB RAEB-t CMML |
12 (13) 7 (8) 47 (53) 17 (19) 6 (7) |
12 (15) 4 (5) 43 (53) 14 (17) 8 (10) |
Patients randomized to the Dacogen arm received Dacogen intravenously infused at a dose of 15 mg/m2 over a 3-hour period, every 8 hours, for 3 consecutive days. This cycle was repeated every 6 weeks, depending on the patient’s clinical response and toxicity. Supportive care consisted of blood and blood product transfusions, prophylactic antibiotics, and hematopoietic growth factors. The study endpoints were overall response rate (complete response + partial response) and time to AML or death. Responses were classified using the MDS International Working Group (IWG) criteria; patients were required to be RBC and platelet transfusion independent during the time of response. Response criteria are given in Table 5:
| *Cheson BD, Bennett JM, et al. Report of an International Working Group to Standardize Response Criteria for MDS. Blood. 2000; 96:3671-3674. | ||
| Complete Response (CR)
≥ 8 weeks |
Bone Marrow | On repeat aspirates:
|
| Peripheral Blood | In all samples during response:
|
|
| Partial Response (PR)
≥ 8 weeks |
Bone Marrow | On repeat aspirates:
|
| Peripheral Blood | Same as for CR | |
The overall response rate (CR+PR) in the ITT population was 17% in Dacogen-treated patients and 0% in the SC group (p<0.001). (See Table 6) The overall response rate was 21% (12/56) in Dacogen-treated patients considered evaluable for response (i.e., those patients with pathologically confirmed MDS at baseline who received at least 2 cycles of treatment). The median duration of response (range) for patients who responded to Dacogen was 288 days (116-388) and median time to response (range) was 93 days (55-272). All but one of the Dacogen-treated patients who responded did so by the fourth cycle. Benefit was seen in an additional 13% of Dacogen-treated patients who had hematologic improvement, defined as a response less than PR lasting at least 8 weeks, compared to 7% of SC patients. Dacogen treatment did not significantly delay the median time to AML or death versus supportive care.
| Parameter | Dacogen N=89 |
Supportive Care N=81 |
|---|---|---|
| **p-value <0.001 from two-sided Fisher's Exact Test comparing Dacogen vs. Supportive Care. †In the statistical analysis plan, a p-value of ≤ 0.024 was required to achieve statistical significance. |
||
| Overall Response Rate (CR+PR)† | 15 (17%)** | 0 (0%) |
| Complete Response (CR) | 8 (9%) | 0 (0%) |
| Partial Response (PR) | 7 (8%) | 0 (0%) |
| Duration of Response | ||
| Median time to (CR+PR) response - Days (range) | 93 (55-272) | NA |
| Median Duration of (CR+PR) response - Days (range) | 288 (116-388) | NA |
All patients with a CR or PR were RBC and platelet transfusion independent in the absence of growth factors.
Responses occurred in patients with an adjudicated baseline diagnosis of AML.
14.2 Single-arm Studies
Three open-label, single-arm, multicenter studies were conducted to evaluate the safety and efficacy of Dacogen in MDS patients with any of the FAB subtypes. In one study conducted in North America, 99 patients with IPSS Intermediate-1, Intermediate-2, or high risk prognostic scores received Dacogen by intravenous infusion at a dose of 20 mg/m2 IV over 1-hour daily, on days 1-5 of week 1 every 4 weeks (1 cycle). The results were consistent with the results of the controlled trial and summarized in Table 8.
|
Demographic or Other Patient Characteristic |
Dacogen |
|
Age (years) |
|
|
Gender n (%) |
|
|
Race n (%) |
|
|
Days From MDS Diagnosis to First Dose |
|
|
Previous MDS Therapy n (%) |
|
|
RBC Transfusion Status n (%) |
|
|
Platelet Transfusion Status n (%) |
|
|
IPSS Classification n (%) |
|
|
FAB Classification n (%) |
|
|
Parameter |
Dacogen |
|
Overall Response Rate (CR+PR) |
16 (16%) |
|
Duration of Response |
|
| + indicates censored observation * Cheson BD, Bennett JM, et al. Report of an International Working Group to Standardize Response Criteria for MDS. Blood. 2000; 96:3671-3674. |
|
15 REFERENCES
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- American Society of Health-System Pharmacists. ASHP Guidelines on Handling Hazardous Drugs: Am J Health-Syst Pharm. 2006;63:1172-1193.
- Polovich M., White JM, Kelleher LO (eds). Chemotherapy and biotherapy guidelines and recommendations for practice (2nd ed.) 2005. Pittsburgh, PA: Oncology Nursing Society.
16 HOW SUPPLIED/STORAGE AND HANDLING
NDC 62856-600-01, 50 mg single-dose vial individually packaged in a carton.
Storage
Store vials at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
17 PATIENT COUNSELING INFORMATION
Instructions for Patients
Women of childbearing potential should be advised to avoid becoming pregnant while receiving treatment with Dacogen and for I month afterwards, and to use effective contraception during this time, [See Warnings and Precautions (5.3)].
Men should be advised not to father a child while receiving treatment with Dacogen, and for 2 months afterwards. During these times, men with female partners of childbearing potential should use effective contraception [See Warnings and Precautions (5.4) and Nonclinical Toxicology (13.1)].
Patients should be advised to monitor and report any symptoms of neutropenia, thrombocytopenia, or fever to their physician as soon as possible [See Warnings and Precautions (5.1)].
Eisai Inc.
Manufactured by Pharmachemie B.V. Haarlem, The Netherlands
Manufactured for Eisai Inc., Woodcliff Lake, NJ 07677
Dacogen® is a registered trademark of Astex Pharmaceuticals, Inc.,
Dublin, CA, U.S.A. used under license.
PRINCIPAL DISPLAY PANEL
NDC 62856-600-01
DACOGEN®
decitabine for injection
50 mg per vial
FOR INTRAVENOUS USE ONLY
WARNING: Cytotoxic Agent
Single use sterile vial
Rx ONLY

Dacogendecitabine INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||