CYTRA
CYTRA-3 SYRUP
FULL PRESCRIBING INFORMATION: CONTENTS*
- CYTRA-3 SYRUP
- CLINICAL PHARMACOLOGY
- CYTRA INDICATIONS AND USAGE
- CYTRA CONTRAINDICATIONS
- WARNINGS AND PRECAUTIONS
- CYTRA ADVERSE REACTIONS
- OVERDOSAGE
- CYTRA DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- STORAGE
- PRODUCT PACKAGING
FULL PRESCRIBING INFORMATION
CYTRA-3 SYRUP
Rx OnlyNDC 60258-002-16
DESCRIPTION
3
Inactive Ingredients:
Potassium Citrate Monohydrate
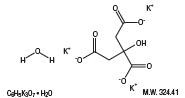
Sodium Citrate Dihydrate
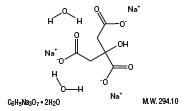
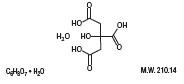
Citric Acid Monohydrate
CLINICAL PHARMACOLOGY
CYTRA INDICATIONS AND USAGE
CYTRA CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
CYTRA ADVERSE REACTIONS
OVERDOSAGE
CYTRA DOSAGE AND ADMINISTRATION
Usual Adult Dosage:
Usual Pediatric Dosage:
Usual Dosage Range:
SHAKE WELL BEFORE USING
HOW SUPPLIED
STORAGE
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSAGE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
PRODUCT PACKAGING
NDC 60258-002-16
CYTRA-3 SYRUP
A SUGAR-FREE SYSTEMIC ALKALIZER
EACH TEASPOONFUL (5 mL) CONTAINS:
Each mL contains 1 mEq Potassium Ion and 1 mEq Sodium Ion, and
is equivalent to 2 mEq Bicarbonate (HCO3).
Rx Only
16 fl oz (473 mL)
INDICATIONS AND USAGE:
DOSAGE AND ADMINISTRATION: SHAKE WELL BEFORE USING.
See attached insert for full prescribing information.
STORAGE:
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSAGE,
SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.



CYTRAPotassium Citrate Monohydrate, Sodium Citrate Dihydrate, Citric Acid Monohydrate SYRUP
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!